Surgical Management of Nondysraphic Giant Thoracic Intramedullary Lipoma: A Case Report by Duval Dario Molina Chóez in Techniques in Neurosurgery & Neurology_journal of neurosurgery spine impact factor
Abstract
Background: Spinal cord intramedullary lipomas are rare and commonly associated with spinal dysraphism and account for 2% of intramedullary tumors, while lipomas not associated with spinal dysraphism are even less frequent, accounting for 1% of cases. Most of the intradural lipomas are subpial and not intramedullary.
Objective: We aim to provide a summary of the pathology, clinical presentation, and management strategies of true giant thoracic intramedullary lipoma without dysraphism with review of the literature.
Case presentation: We report a case of a patient with giant thoracic intramedullary lipoma who presented with paraparesis and bladder dysfunction. There were not imaging studies that evidenced dysraphism. The patient underwent surgery and diagnosis was confirmed histopathological. Postoperatively, neurological symptoms improved in this case.
Keywords: Intramedullary Lipoma; Thoracic Spine Lipoma; Dysraphism
Abbreviations: MRI: Magnetic Resonance Imaging; CUSA: Cavitron Ultrasonic Surgical Aspirator; Gad: Gadolinium
Introduction
Spinal lipomas are typically extramedullary lesions, and classically found in the lumbosacral spine with spinal dysraphism. Most of the intradural lipomas are subpial and not intramedullary. Truly intramedullary spinal cord lipomas are rare and account for less than 1% of all spinal cord lesions [1]. Spinal cord lipomas are frequently associated with spinal dysraphism, such as spina bifida, lip myelomeningocele, myelomeningocele, diastematomyelia, cutaneous lipoma and others [2]; and the other hand the lipoma without spinal dysraphism have been reported to be only 1% of all spinal cord lipomas [3].
Case Presentation
We present a case of a 17-year-old female, previously healthy who is admitted with a history of lower limb weakness of 1 year of evolution that blocks gait and bladder dysfunction characterized by urinary incontinence. Neurological examination showed 1/5 lower limb weakness, hyperreflexia, hypoesthesia from the T8 medullary level, sign of Babinski. MRI showed an elongated and ovoid intramedullary lesion with evident medullary widening from T3 to T7. The intramedullary lesion arose from the posterior aspect of the thoracic spinal cord. On T1- weighted images the lesion was hyperintense and on fast spin echo T2-weighted images the lesion was partially hyperintense. With the gadolinium administration, the lesion showed intense enhancement. On short tau inversion recovery sequence images, the lesion appeared profoundly hypointense Figure 1. We performed a microsurgical resection of the lesion. The patient was placed in the ventral position. We used electromyography and evoked potentials during the procedure. We performed laminectomy from T3 to T7 to expose the poles of the lesion and performed a longitudinal durotomy on the midline. Under microscopic vision, a myelotomy was performed on the midline and we used CUSA to perform a devastation of the lesion until to reach the limits of the anterior cords; simultaneously using bipolar coagulation and micro scissors. Once considerable devastation had been performed, the portion adhered to the anterior spinal cords was partially resected, avoiding direct bipolar coagulation. Finally, a partial resection of the tumor of approximately 70% was achieved. Surgical hemostasis was carefully verified. A Dural closure was completed leaving a wide spinal canal. We perform laminoplasties of the operated levels Figure 2. During the immediate postoperative period, there were no clinical changes, however, after the first month, the patient presented improvement in the strength of both legs, achieving assistance walking. At the 6-month follow-up, the patient showed walking without support and urinary sphincter control. Histopathological analysis reported a lesion compatible with lipoma Figure 3. MRI at 1 year follow up showed residual lesion Figure 4.
Figure 1: (A)(B) Sagittal (C) Axial T1 weighted image. There is a hyperintense intramedullary ovoid and fusiform lesion. The lesion spans along five vertebral bodies at level of T3-T7 (arrows). (D) Sagittal T2 weighted image. The lesion demonstrates high signal intensity, consistent with fat component. (E) Sagittal post Gad T1 weighted image. There is a moderate enhancement of the lesion. (F) Sagittal STIR. The lesion exhibit very low signal (hypointense), confirming fat content.

Figure 2: (A) Exposed tumor through laminectomies of levels T3-T7 and longitudinal durotomy. A Fat-looking tumor is observed. (B) Myelotomy in the midline exposing the tumor inside. (C) Subtotal tumor resection (piece). (D) Residual tumor covering healthy spinal cord. (E) Laminoplasties.
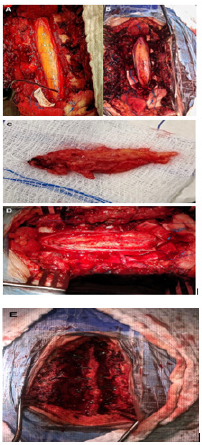
Figure 3: Hematoxylin eosin. (A) 20x. Panoramic image showing mature adipocytes with fine connective tissue partitions and bleeding areas. (B) 40x. Magnification where adipocytes with benign cytological characteristics are seen in more detail.
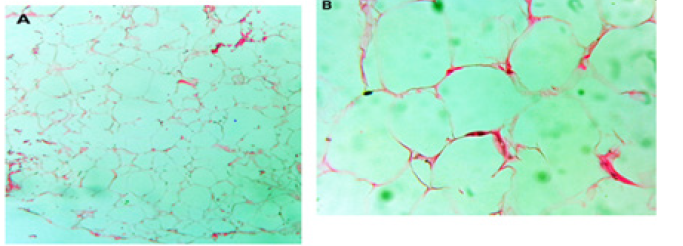
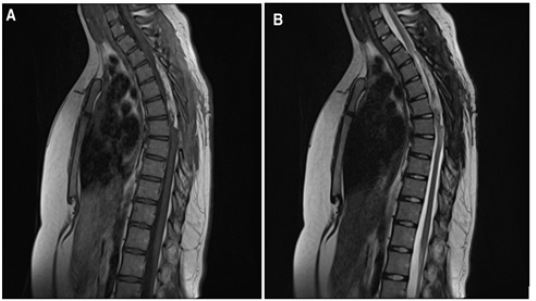
Figure 4: Residual tumor seen in (A) Sagittal post Gad T1 weighted image and (B) Sagittal T2 weighted image.
Discussion
Spinal cord lipomas are rare lesions, representing less than 1% of all spinal cord tumors [1]. They are typically extradural in location and are predominantly found at the sacral/lumbosacral regions, in conjunction with spinal dysraphism and subcutaneous lipomas [4,5]. Intradural and intramedullary lipomas are even less frequent. Because of the critical location, intramedullary cervical tumors often carry a poor prognosis [5,6]. Spinal cord intramedullary lipomas are more often in young people, especially in the second and third decades of life. Males and females are equally affected. The classical location of these tumors is intradural and they may be intramedullary, extramedullary or a combination of the two [7]. Many theories have been proposed for the origin of these lesions. “Developmental error theory” is the most accepted. According to this theory, embryologic inclusion of misplaced adipocytes during the formation of the neural tube closure gives rise to lipomas and explain the dorsal location of these lesions as well as that some spinal lipomas are unassociated with dysraphism [8-10]. Histologically, spinal cord intramedullary lipomas consist of mature fat cells, with intervening collagen fibers; admixed nerves bundles are often located at the periphery, suggestive of secondary entrapment of adjacent nerve roots [8]. Patients with spinal cord intramedullary lipomas usually present with a slow, indolent, progressive deterioration of neurologic function with hypotonia, leg weakness, loss of sense position and gait disturbance. Most patients have progressive impairment of the lower extremity, bladder, or bowel functions [8,11]. MRI is considered the gold standard for the identification and the differential diagnosis of lipomas. Additionally, due to its multiplanar imaging capability, it can provide valuable preoperative information. MRI not only confirms the fat component of the tumor, but it also delineates its relationship with the adjacent structures, which is important for surgical planning. High signal intensity lesions on T1-weighted images are quite characteristic of lipomas due to its very short relaxation time of the fat. Fat-suppressed images confirming the presence of fat. Post gadolinium images do not reveal internal enhancement of the lesion [12]. In our case the lesion showed a moderate enhancement with gadolinium. Treatment of intramedullary lipoma is controversial and depends on symptoms. Indications for surgery and the surgical strategies for treatment are still discussed controversially. In asymptomatic patients no treatment is required, while in symptomatic patients’ surgical decompression is sufficient. Early surgical decompression prevents irreversible spinal cord dysfunction because most symptomatic patients usually do not substantially improve after surgery. Therefore, the surgical planning should be decompression before symptom progression. These lesions are benign; however complete surgical removal is virtually impossible due to their vast attachment to the surrounding neural tissues [6,8,9,13,14]. In our case the lesion was removed partially due to the infiltrative nature of the lesion over the spinal cord and avoid damaging the vascularity. This shows that aggressive surgical removal may lead to spinal cord damage and worsening of neurological symptoms. Recent technical advances, like the ultrasonic aspirator, surgical laser and operating microscope have significantly improved surgical outcomes for patients with intraspinal lipomas.
The ultrasonic aspirator and surgical laser can be used for gentle and careful debulking of these tumors [13,15,16]. In most cases additional decompression of neural structures by laminectomy or laminoplasty is also necessary to relieve the neurological symptoms. Laminectomies should be preferred after partial resection of the lipomas located at the thoracic levels, which are the more stable segments and if the patients is not young [15], however in our case we preferred laminoplasties to achieve spinal canal diameter expansion and to ensure a longer active life expectation, due to the patient’s very young age. Prognosis depends on the extent of the lesion and the extent of neurological dysfunction. Early diagnosis and treatment provide a better outcome. Concerning symptomatic patients, 40% show motor improvement after surgery [8,17]. The goal of the surgery in these tumors is the decompression, a partial tumor removal can achieve neurological improvement. In the case we present, the importance of performing planned and technically methodical surgical management is detailed.
Acknowledgement
Maria Augusta Carrillo Chóez
References
- Caram PC, Carton CA, Scarcella G (1957) Intradural lipomas of the spinal cord with emphasis on the intramedullary lipomas. Journal of Neurosurgery 14(1): 28-42.
- Shen SH, Lirng JF, Chang FC, Lee JY, Luo CB, et al. (2001) Magnetic resonance imaging appearance of intradural spinal lipoma. Chinese Medical Journal 64(6): 364-368.
- Bhatoe HS, Singh P, Chaturvedi A, Sahai K, Dutta V, et al. (2005) Nondysraphic intramedullary spinal cord lipomas: a review. Neurosurgical Focus 18(2): ECP1.
- Rogers HM, Long DM, Chou SN, French LA (1971) Lipomas of the spinal cord and cauda equina. Journal of Neurosurgery 34(3): 349-354.
- Severino R, Severino P (2017) Cervical intramedullary spinal cord lipoma. Surgical Neurology International 8: 255.
- Lee M, Rezai AR, Abbott R, Coelho DH, Epstein FJ (1995) Intramedullary spinal cord lipomas. Journal of Neurosurgery 82(3): 394-400.
- Finn MA, Walker ML (2007) Spinal lipomas: clinical spectrum, embryology, and treatment. Neurosurgical Focus 23(2): E10.
- Solomou A, Panagiotopoulos V, Kraniotis P, Apostolopoulou, K, Tzortzidis F (2017) Massive bilocular spinal cord intramedullary lipoma of the thoracic spine. BJR 3(4): 20170009.
- Dyck P (1992) Intramedullary lipoma diagnosis and treatment. Spine 17(8): 979-981.
- Corr P, Beningfield SJ (1989) Magnetic resonance imaging of an intradural spinal lipoma: a case report. Clinical Radiology 40(2): 216-218.
- Blount JP, Elton S (2001) Spinal lipomas. Neurosurgical Focus 10(1): e3.
- Kamat, A, Findlay G (2003) Intramedullary migration of spinal cord lipoma. BMJ 74(11): 1593-1594.
- Razack N, Jimenez OF, Aldana P, Ragheb J (1998) Intramedullary holocord lipoma in an athlete: case report. Neurosurgery 42(2): 394-397.
- Rogers HM, Long DM, Chou SN, French LA (1971) Lipomas of the spinal cord and cauda equina. Journal of Neurosurgery 34(3): 349-354.
- Arslan E, Kuzeyli K, Acar Arslan E (2014) Intraspinal lipomas without associated spinal dysraphism. Iranian Red Crescent Medical Journal 16(5): e11423.
- Kodama T, Numaguchi Y, Gellad FE, Sadato N (1991) Magnetic resonance imaging of a high cervical intradural lipoma. Computerized medical imaging and graphics. Computerized Medical Imaging Society 15(2): 93-95.
- McLone DG, Naidich TP (1986) Laser resection of fifty spinal lipomas. Neurosurgery 18(5): 611-615.
Publishers: https://crimsonpublishers.com/




No comments:
Post a Comment