Assessment of Role of Platelet-to-Lymphocyte Ratio in Prediction of Angiographic No-Reflow in Patients Subjected to Primary Percutaneous Coronary Intervention by Ghada Mahmoud Shawkat Ali in Open Journal of Cardiology & Heart Diseases_heart disease research articles
Abstract
Primary Percutaneous Intervention is the most advantageous and rewarding reperfusion strategy
available in patients with acute ST-segment–elevation myocardial infarction (STEMI), although it fails
to restore optimal myocardial reperfusion in a sizeable portion of patients mostly because of no-reflow
phenomenon.
No reflow is defined as suboptimal myocardial reperfusion through a part of coronary circulation without
angiographic evidence of mechanical vessel obstruction. Inflammation has a substantial role in the
initiation and propagation of the atherosclerotic process. The platelet–lymphocyte ratio (PLR) has been
recently proposed to be a marker of thrombotic and inflammatory state, mainly in patients with coronary
or peripheral ischemic events.
Abbreviations: PPCI: Primary Percutaneous Coronary Intervention; PCI: Percutaneous Coronary Intervention; STEMI: Segment Elevation Myocardial Infarction; PLR: Platelet to Lymphocyte Ratio; TIMI: Thrombolysis in Myocardial Infarction
Introduction
Myocardial infarction occurs when myocardial ischemia, a diminished blood supply to the
heart, exceeds a critical threshold and overwhelms myocardial cellular repair mechanisms
designed to maintain normal operating function and homeostasis Critical myocardial ischemia
can occur as a result of increased myocardial metabolic demand, decreased delivery of oxygen
and nutrients to the myocardium via the coronary circulation, or both. An interruption in
the supply of myocardial oxygen and nutrients occurs when a thrombus is superimposed on
an ulcerated or unstable atherosclerotic plaque and results in coronary occlusion [1]. The
moment a STEMI is diagnosed, treatment is begun. In addition to administering several drugs
to attempt to stabilize the heart muscle - including oxygen, morphine, beta blockers and statin
- steps are taken immediately to open up the blocked artery [2,3]. Primary percutaneous
coronary intervention (PPCI) is more effective than thrombolytic therapy for the treatment of
ST-segment elevation myocardial infarction (STEMI) when delivered by an experienced team
soon after the onset of symptoms. However, performance of PCI in a timely fashion can be
logistically challenging, and delays in time to reperfusion with primary PCI adversely affect
outcome [4].
No reflow is defined as suboptimal myocardial reperfusion through a part
of coronary
circulation without angiographic evidence of mechanical vessel
obstruction. During the last 3
decades, multiple experimental and clinical studies have identified a
number of predisposing
factors of no-reflow phenomenon and have proposed an array of
explanative mechanisms
and strategies to overcome it in the clinical setting, however, several
aspects of no-reflow
phenomenon remain poorly understood [5-7]. Inflammation has a
substantial role in the initiation and propagation of the
atherosclerotic process. The
platelet–lymphocyte ratio (PLR) has been recently proposed to
be a marker of thrombotic and inflammatory state, mainly in
patients with coronary or peripheral ischemic events. However,
little is known regarding the PLR and its association with adverse
outcomes in patients with cardiovascular diseases [8-10]. Thus, it
is interesting to assess the predictive value of pre-procedural PLR
in the development of no reflow in patients undergoing coronary
stent implantation for the treatment of STEMI [10].
Role of Thrombosis in STEMI
The most common cause of a heart attack is a blood clot that forms inside a coronary artery, or one of its branches. This blocks the blood flow to a part of the heart.
Coronary artery plaques and thrombosis
Figure 1:Plaque rupture and healing: Rupture of a thin cap fibroatheroma with nonfatal thrombus and subsequent healing with fibrous tissue formation and constrictive remodeling [11,12].
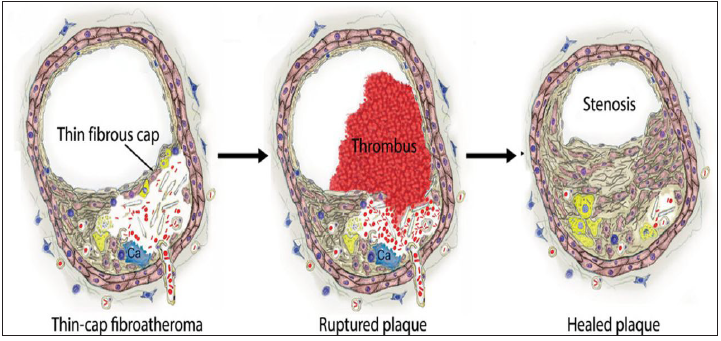
A. Mechanisms of plaque rupture, erosion, and thrombosis: In plaque rupture, a structural defect-a gap-in the fibrous cap exposes the highly thrombogenic core to the blood Dislodged plaque material is sometimes found within the thrombus, indicating that rupture and thrombosis coincided and thereby supporting its causal relationship (Figure 1).
B. Mechanisms of plaque rupture: Plaque rupture occurs where the cap is thinnest and most infiltrated by foam cells (macrophages) [11]. In eccentric plaques, the weakest spot is often the cap margin or shoulder region, [12] and only extremely thin fibrous caps are at risk of rupturing. Rupture of a thin cap and subsequent thrombosis may occur spontaneously, but in some cases, a temporary increase in emotional or physical stress provides the final triggering of the event.
C. Thrombosis: The magnitude of the thrombotic response
on ruptured or eroded plaques is extremely variable, and only
occasionally a major and life-threatening luminal thrombus evolve.
Probably the determinants are those of the classic triad of Virchow:
a. thrombogenicity of the exposed plaque material
b. local flow disturbances
c. systemic thrombotic propensity.
With plaque rupture, cap collagen and the highly thrombogenic
lipid core, enriched in tissue factor–expressing apoptotic micro
particles, are exposed to the thrombogenic factors of the blood
[13,14]. Although blood flow continues over the culprit lesion,
micro-emboli of plaque material and thrombus may be washed
away, leading to distal embolization [15,16] that may cause
microvascular obstruction and prevention of myocardial perfusion
despite a re-canalized infarct-related coronary artery.
Myocardial ischemic events
Myocardial injury and myocardial cell death: Myocardial ischemia happens if blood supply to the myocardium does not meet the demand. If this imbalance persists, it triggers a cascade of cellular, inflammatory and biochemical events, leading eventually to the irreversible death of heart muscle cells, resulting in MI.
Evolution of MI and ventricular remodeling: Typical MI initially manifests as coagulation necrosis that is ultimately followed by a healing process characterized by formation of myocardial scarring, known as myocardial fibrosis. This mechanism allows significant architectural changes to the composition, shape and contractile function of the myocardium, especially in the left ventricle, which is the major contributor to the contractile function of the heart. Eventually the left ventricle dilates and changes to a more spherical shape, in a process known as ventricular remodeling. Despite being an irreversible process, ventricular remodeling is a regulated process, therefore, specific treatment strategies and agents should be used in acute MI management in order to reduce the occurrence and severity of ventricular remodeling [17].
Primary Percutaneous Coronary Intervention
Primary percutaneous coronary intervention (PPCI) has been shown to be superior to fibrinolysis in the treatment of acute myocardial infarction with ST-segment elevation in patients admitted to highly experienced angioplasty centers, primary angioplasty is offered only to the limited number of patients admitted directly to hospitals with interventional services. Transportation from the local hospital to an angioplasty centre has been considered to represent a major limitation on the widespread use of primary angioplasty (Figure 2).
Figure 2:Thrombotic occlusion of an artery associated with acute myocardial infarction and subsequent Percutaneous Coronary Intervention (PCI). In an occluded infarct-related artery (Panel A), reperfusion can be achieved by the standard means of primary PCI (Panel B) or by the new method of thrombus aspiration (Panel C), followed by stenting [18].

No Reflow Phenomenon after Primary PCI
According to Kloner et al. [5] no-reflow is defined as suboptimal myocardial reperfusion through a part of coronary circulation without angiographic evidence of mechanical vessel obstruction. The concept of “no reflow” refers to a state of myocardial tissue hypo perfusion in the presence of a patent epicardial coronary artery. The underlying cause of no reflow is microvascular obstruction, which may be produced by various mechanisms. No reflow occurs after primary PCI may be asymptomatic or may present clinically with continued chest pain and ST-segment elevation. Reperfusion no reflow is preceded by ischemic cell injury, is confined to the irreversibly damaged necrotic zone, and may be exacerbated at the time of reperfusion. Reperfusion no reflow is an independent predictor of adverse clinical outcome after AMI regardless of infarct size and is associated with heart failure and increased mortality [18,19].
Diagnosis of no reflow
a. ECG ST-segment resolution is a readily available marker
of tissue-level reperfusion, persistence of ST-segment elevation in
an AMI patient may reflect either epicardial artery occlusion or
microvascular obstruction.
b. Coronary angiography allows a semi quantitative grading
of epicardial coronary flow according to the Thrombolysis in
Myocardial Infarction (TIMI) flow grades.
The no-reflow phenomenon is recognized angiographically in
>20% of patients undergoing primary angioplasty for AMI and in
<2% of elective PCI cases. Reduced coronary flow after primary
angioplasty (TIMI flow 0 to 2) is associated with worse outcome
than normal (TIMI 3) flow, even when no significant epicardial
obstruction remains [19]. The TIMI frame count assesses the
number of angiographic frames required for the contrast medium
to reach standardized distal landmarks of the coronary tree, and the
myocardial blush grade is a quantitative assessment of myocardial
contrast density.
TIMI Flow Grading
TIMI Grade Flow’ is a scoring system from 0-3 referring
to levels of coronary blood flow assessed during percutaneous
coronary angioplasty [20-23]:
1. TIMI 0 flow (no perfusion) refers to the absence of any
antegrade flow beyond a coronary occlusion.
2. TIMI 1 flow (penetration without perfusion) is faint
antegrade coronary flow beyond the occlusion, with incomplete
filling of the distal coronary bed.
3. TIMI 2 flow (partial reperfusion) is delayed or sluggish
antegrade flow with complete filling of the distal territory.
4. TIMI 3 is normal flow which fills the distal coronary bed
completely
c. Myocardial contrast echocardiography has greatly
advanced the noninvasive assessment of myocardial perfusion and
may demonstrate microvascular no reflow even among patients
with angiographic TIMI 3 flow after primary PCI, which predicts
worse outcome [24]. Tissue hypo enhancement on contrastenhanced
MRI and CT reflects impaired myocardial perfusion and
correlates with histological evidence of microvascular obstruction.
d. A rise in serum cardiac biomarkers after PCI reflects
myocardial necrosis secondary to tissue hypo perfusion and
ischemia. More than 70% of patients may exhibit elevated troponin
values after an otherwise successful elective PCI [25].
Pathophysiology of no reflow
After prolonged cessation of coronary occlusion and restoration
of blood flow to the epicardial coronary arteries, there is sufficient
structural damage to the microvasculature to prevent restoration
of normal blood flow to the cardiac myocytes. This may lead to
inadequate healing of the cardiac scar. In addition, it may prevent
the development of future collateral flow.
1. It is more pronounced with longer periods of coronary
occlusions. No reflow appears to be a process rather than an
immediate event that occurs at the moment of reperfusion.
2. Microscopic examination showed that the cardiac cells
within the no-reflow area were swollen. The capillary endothelium
was damaged and exhibited areas of regional swelling with large
intraluminal protrusions that in some cases appeared to plug the
capillary lumen [26].
3. Intravascular plugging by fibrin or platelets may also
contribute to the no-reflow phenomenon [27-30]. Beneficial
effects of ibuprofen, prostaglandin E1, and vascular washout with
heparinized saline support the concept that these blood elements
may be important [31-33].
4. Leukocyte intravascular plugging appears to play an
important role in the pathophysiology of no reflow. Engler et al
showed that the no-reflow areas had evidence of capillary leukocyte
plugging [34].
5. Leukocytes may interfere with blood flow by mechanical
plugging and perhaps by their release of oxygen free radicals
that will add further injury to the capillary endothelium [34-37].
Thus, the no-reflow phenomenon is likely multifactorial. During
the ischemic phase, endothelial damage, including endothelial
swelling and myocyte edema, led to initial no-reflow zones. With
reperfusion, additional edema, myocyte contraction, platelets,
fibrin, and leukocyte plugging resulted in expansion of the noreflow
zones over the early hours of reperfusion. Platelet and
leukocyte depletion and vasodilators appeared to lessen no reflow
[38-41].
An additional mechanism plays a very important role during
short-term intervention in acute myocardial infarction. Microemboli
of atherosclerotic debris, blood clots, and platelet plugs are
released into the microcirculation, particularly with restoration of
normal blood flow by thrombolysis, angioplasty, stenting, or other
percutaneous intervention. Although this is more common in vein
graft intervention, it is to be expected in native coronary arteries.
A variety of new, innovative devices are now in clinical practice
and in the research phase to filter these micro-emboli during the
interventional procedure (Figure 3).
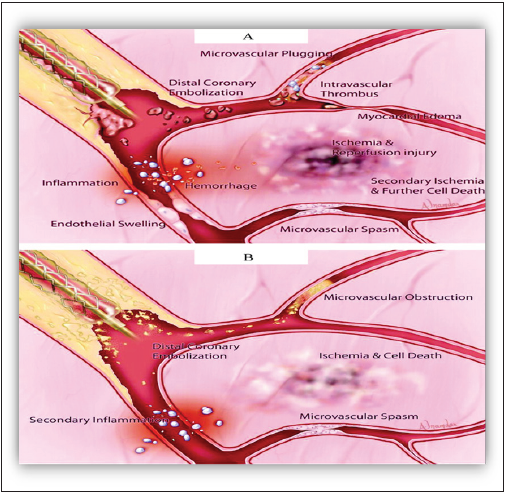
Figure 3:Schematic representation of pathophysiological mechanisms that may contribute to reperfusion no reflow in the setting of primary angioplasty for AMI. The vasculature within the necrotic zone is subjected to additional injury after reperfusion. Microvascular spasm and plugging, intravascular thrombus, endothelial swelling, and capillary compression by edema within the adjacent myocardial tissue may lead to microvascular obstruction. Angioplasty-induced distal coronary embolization of plaque and thrombus may compound the vascular obstruction. An inflammatory response may exacerbate this process, which leads to further myocardial ischemia and cell death. B, Interventional no reflow after no infarct angioplasty is induced by distal coronary embolization of plaque components. Mechanical obstruction of the microvasculature may be accompanied by an inflammatory vascular response that leads to vascular spasm. These mechanisms result in myocardial ischemia and cell death [41].
Platelet - Lymphocyte Ratio (PLR)
Rapid restoration of coronary blood flow to the jeopardized myocardium is the crux of therapy, after acute myocardial infarction. The invention and usage of stents have made PCI a safe, effective, and preferred reperfusion modality for the treatment of STEMI [42]. Nevertheless, even after patency of an infarcted artery was successfully achieved via stent implantation, sufficient myocardial reperfusion was not observed in 2.3% to 29% of patients in the setting of acute myocardial infarction, which is often called the noreflow phenomenon [5,43-45]. Despite the mechanical opening of the infarct-related artery (IRA), early post infarction complications, adverse left ventricular remodeling, and in-hospital and long-term morbidity and mortality are increased in patients who develop no reflow [46-49]. The platelet–lymphocyte ratio (PLR) has been recently proposed to be a marker of thrombotic and inflammatory state [8,9]. However, little is known regarding the PLR and its association with adverse outcomes in patients with cardiovascular diseases [10].
Significance of PLR
Acute coronary syndrome (ACS) is a leading cause of
morbidity and mortality. Inflammation has an important role in
the progression and destabilization of coronary atherosclerosis.
Several inflammatory biomarkers, such as interleukin (IL)-6,
[50] C-reactive protein [51,52], and matrix metalloproteinases
[53], have been identified as independent predictors of adverse
outcomes in patients with ACS. However, these biomarkers are not
used as routine or inexpensive examinations for patients with ACS.
Increased platelet activation has an important role in the
initiation and progression of atherosclerosis [54]. Higher platelet
counts may reflect increased platelet activation, which has a
pivotal role in megakaryocytic proliferation and produces relative
thrombocytosis [55]. In addition, low lymphocyte counts indicate
a depressed immune response that is associated with adverse
outcomes in cardiovascular disorders [56,57]. Therefore, an
elevated platelet-to-lymphocyte ratio (PLR) might enhance microparticle,
platelet, monocyte, or neutrophil aggregate production,
which results in a state of activated hemostasis, and it will lead
to an increased risk of adverse outcomes in ACS. Therefore, PLR
is a combined marker of lymphocytopenia and thrombocytosis
and may better indicate no-reflow as well as mortality. Increased
platelet counts may reflect underlying inflammation as several
inflammatory mediators stimulate megakaryocytic proliferation
and produce relative thrombocytosis.
Activated platelets release inflammatory and mitogenic
substances into the local microenvironment, which would promote
the recruitment of more platelets and leukocytes [58,59]. The rush
of platelets and neutrophils that follows reperfusion may lead
to the formation of neutrophil-platelet aggregates that plug the
microcirculation and reperfusion-related injury [60]. A positive
correlation was found between the acute phase reactants and proinflammatory
proteins (CRP, interleukin (IL)-1, IL-6, and tumor
necrosis factor a) and an elevated platelet count in nonspecific
inflammatory conditions [61]. In addition, recent studies have
shown that patients with CAD have increased platelet and
monocyte aggregates in their bloodstream, which was associated
with plaque instability, worse in-hospital outcomes, and increased
risk of future cardiac events [62,63]. On the other hand, elevated
numbers of lymphocytes have also been speculated to be related
to an increase in plaque stability [64]. Previous studies reported
that lymphocytopenia was independently related to mechanical
complications and mortality in patients with acute myocardial
infarction.
Conclusion
Although several mechanisms have been proposed to explain the no reflow phenomenon, the pathophysiology of no-reflow has not been fully explained, and its Aetiology appears to be multifactorial. Platelet lymphocyte ratio (PLR) is a novel factor that can predict none-reflow in patients subjected to primary percutaneous intervention and can predict the post interventional adverse cardiac outcomes, complications and mortality. Further large-scale, prospective and multicenter studies are needed to clarify and confirm the association between the PLR and no reflow, which might be promising in identifying patients who need prophylactic treatment.
References
- Da Costa PE (1994) Robbins pathologic basis of disease. In: Cotran RS (Ed.), (5th edn), Philadelphia, USA.
- Amsterdam E, Brindis RG, Wenger K, Donald EC, Theodore GG, et al. (2014) AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 130 (25): 2354-2394.
- Pollack CV Jr, Diercks DB, Roe MT, Peterson ED (2005) American College of Cardiology/American Heart Association guidelines for the management of patients with ST-elevation myocardial infarction: Implications for emergency department practice. Ann Emerg Med 45 (4): 363-376.
- Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 361(9351): 13-20.
- Kloner RA, Ganote CE, Jennings RB (1974) The no-reflow phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54(6): 1496-1508.
- Reffelmann T, Kloner RA (2006) The no-reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol 101(5): 359-372.
- Rezkalla SH, Kloner RA (2008) Coronary no-reflow phenomenon: From the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv 72(7): 950-957.
- Wang D, Yang JX, Cao DY, Wan XR, Feng FZ, et al. (2013) Preoperative neutrophil-lymphocyte and platelet-lymphocyte ratios as independent predictors of cervical stromal involvement in surgically treated endometrioid adenocarcinoma. OncoTargets Ther 6: 211-216.
- Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, et al. (2008) Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg 12(8): 1422-1428.
- Azab B, Shah N, Akerman M, McGinn JT (2012) Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis 34(3): 326-334.
- Virmani R KF, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20: 1262-1275.
- Falk E (1983) Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br Heart J 50(2): 127-134.
- Fernández Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, et al. (1994) Characterization of the relative thrombogenicity of atherosclerotic plaque components: Implications for consequences of plaque rupture. J Am Coll Cardiol 23: 1562-1569.
- Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, et al. (2011) Microparticles, vascular function, and atherothrombosis. Circ Res 109(5):593-606.
- Falk E (1985) Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 71(4): 699-708.
- Falk E, Thuesen L (2003) Pathology of coronary microembolization and no reflow. Heart 89(9): 983-985.
- Burchfield JS XM, Hill JA (2013) Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 128 (4): 388-400.
- Vetrovec GW (2008) Improving reperfusion in patients with myocardial infarction. New England Journal of Medicine 358 (6): 634-637.
- Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, et al. (2000) Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 36(4):1202-1209.
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, et al. (2000) The TIMI risk score for unstable angina/Non–ST elevation MI. JAMA.
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, et al. (2000) The TIMI risk score for unstable angina/Non–ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 284(7): 835-842.
- TIMI risk score.
- Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, et. al. (2000) TIMI risk score for ST-Elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infracting myocardium early II trial sub study circulation. 102(17): 2031-2037.
- Ito H, Okamura A, Iwakura K, Masuyama T, Hori M, et al. (1996) Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 93(11): 1993-1999.
- Bonz AW, Lengenfelder B, Strotmann J, Held S, Turschner O, et al. (2002) Effect of additional temporary glycoprotein IIb/IIIa receptor inhibition on troponin release in elective percutaneous coronary interventions after pretreatment with aspirin and clopidogrel (TOPSTAR trial). J Am Coll Cardiol 40(2): 662-668.
- Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54(6): 1496-1508.
- Topol EJ, Yadav JS (2000) Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation 101(5): 570-580.
- Seydoux C, Goy JJ, Davies G (1993) Platelet and neutrophil imaging techniques in the investigation of the response to thrombolytic therapy and the no-reflow phenomenon. Am Heart J 125(4): 1142-1147.
- Michaels AD, Gibson CM, Barron HV (2000) Microvascular dysfunction in acute myocardial infarction: Focus on the roles of platelet and inflammatory mediators in the no-reflow phenomenon. Am J Cardiol 85(5A): 50B–60B.
- Cuypers J, Matakas F (1974) The effect of postischemic hyperemia on intracranial pressure and the no-reflow phenomenon. Acta Neuropathol (Berl) 29(1): 73-84.
- Douglas B WH, Song Y, et al. (1987) Beneficial effects of ibuprofen on experimental microvascular free flaps: Pharmacologic alteration of the no-reflow phenomenon. Plast Reconstr Surg 79(3): 366-371.
- Hataya Y, Matsuo K, Ishigaki M, Imai Y, Taki K, et al. (1999) Retrograde intra-arterial infusion of prostaglandin E1 and heparin for the no-reflow phenomenon after oromandibular reconstruction with a free fibular flap. Ann Plast Surg 42(1): 92-95.
- Calhoun KH, Tan L, Seikaly H (1999) An integrated theory of the no-reflow phenomenon and the beneficial effect of vascular washout on no-reflow. Laryngoscope 109(4): 528-535.
- Engler RL, Schmid Schönbein GW, Pavelec RS (1983) Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol 111(1): 98-111.
- Kloner RA, Alker K, Campbell C, Figures G, Eisenhauer A, et al. (1989) Does tissue-type plasminogen activator have direct beneficial effects on the myocardium independent of its ability to lyse intracoronary thrombi? Circulation 79: 1125-1136.
- Koo A, Komatsu H, Tao G, Inoue M, Guth PH, et al. (1991) Contribution of no-reflow phenomenon to hepatic injury after ischemia-reperfusion: evidence for a role for superoxide anion. Hepatology 15(3): 507-514.
- Punch J, Rees R, Cashmer B, Wilkins E, Smith DJ, et al. (1992) Xanthine oxidase: Its role in the no-reflow phenomenon. Surgery 111(2): 169-176.
- Humphrey SM, Gavin JB, Herdson PB (1982) Catecholamine-depletion and the no-reflow phenomenon in anoxic and ischaemic rat hearts. J Mol Cell Cardiol 14(3): 151-161.
- Humphrey SM, Seelye RN, Gavin JB (1982) The influence of adenosine on the no-reflow phenomenon in anoxic and ischaemic hearts. Pathology 14(2): 129-133.
- Westin M, Hedén P (1988) Calcitonin gene-related peptide delays the no-reflow phenomenon in the rat island flap. Ann Plast Surg 21(4): 329-334.
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH, et al. (2008) Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117(24): 3152-3156.
- Luigi PB, Carina BL, Michael AB, Carlo DM, James SK, et al. (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J 33(20): 2569-2619.
- Harrison RW, Aggarwal A, Ou FS, Klein LW, Rumsfeld JS, et al. (2013) Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 111(2): 178-184.
- Magro M, Nauta ST, Simsek C, Boersma E, van der Heide E, et al. (2012) Usefulness of the SYNTAX score to predict ‘‘no reflow’’ in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol 109(5): 601-606.
- Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, et al. (2010) 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 55(21): 2383-2389.
- Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, et al. (2004) Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109(9): 1121-1126.
- Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, et al. (2012) Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 110(5): 621-627.
- Celik T, Kaya MG, Akpek M, Yarlioglues M, Sarli B, et al. (2014) Does serum bilirubin level on admission predict TIMI flow grade and in-hospital MACE in patients with STEMI undergoing primary PCI. Angiology 65(3):198-204.
- Sen N, Afsar B, Ozcan F, Buyukkaya E, Isleyen A, et al. (2013) The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long-term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis 228(1): 203-210.
- Mach F, Schönbeck U, Fabunmi RP, Murphy C, Atkinson E, et al. (1999) T lymphocytes induce endothelial cell matrix metalloproteinase expression by a CD40L-dependent mechanism: Implications for tubule formation. Am J Pathol 154 (1): 229-238.
- Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, et al. (2003) Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation 108(5): 512-515.
- Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, et al. (2008) C-reactive protein in cardiovascular disease. JAMA 294: 2866-2890.
- Davies MJ, Woolf N, Rowles PM, Pepper J (1988) Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br Heart J 60(6): 459-464.
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, et al. (2004) Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 110(14): 2032-2038.
- Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, et al. (2007) Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial). Am J Cardiol 99(8): 1055-1061.
- Acanfora D, Gheorghiade M, Trojano L, Furgi G, Pasini E, et al. (2001) Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J 142(1): 167-173.
- Ommen SR, Gibbons RJ, Hodge DO, Thomson SP (1997) Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 79(6): 812-814.
- Gawaz M, Langer H, May AE (2005) Platelets in inflammation and atherogenesis. J Clin Invest 115(12): 3378-3384.
- Peerschke EI, Yin W, Ghebrehiwet B (2010) Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol Immunol 47(13): 2170-2175.
- Mehta JL, Nichols WW, Mehta P (1988) Neutrophils as potential participants in acute myocardial ischemia: Relevance to reperfusion. J Am Coll Cardiol 11(6): 1309-1316.
- Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, et al. (2003) Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol 26(2): 135-140.
- Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, et al. (1998) Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol 31(2): 352-358.
- Zhang SZ, Jin YP, Qin GM, Wang JH (2007) Association of platelet-monocyte aggregates with platelet activation, systemic inflammation, and myocardial injury in patients with non-ST elevation acute coronary syndromes. Clin Cardiol 30(1): 26-31.
- Zazula AD, Precoma-Neto D, Gomes AM, Kruklis H, Barbieri GF, et al. (2008) An assessment of neutrophils/lymphocytes ratio in patients suspected of acute coronary syndrome. Arq Bras Cardiol 90(1): 31-36.
Publishers: https://crimsonpublishers.com/




No comments:
Post a Comment