Elevated Serum Delta Bilirubin Might Predict the Occurrence of Different Types of Jaundice with Special Reference to Chronicity of the Disease by Subhadip Choudhuri in Developments in Clinical & Medical Pathology_Journal of Medical Pathology
Abstract
Background: Present study was aimed to evaluate whether measurement of serum delta bilirubin and albumin level predict the chronicity and subsequent impaired hepatic function across the spectrum of different types of jaundice.
Methods: Serum total bilirubin, Delta bilirubin, albumin and GGT activity was measured by reflectance spectrophotometry.
Result: Decreased serum albumin was found in a significant inverse correlation with elevated delta bilirubin level among the subjects with obstructive (r=-0.4217, p=0.0023) and hepatic jaundice (r=-0.4214, p=0.0023).
Conclusion: Significant inverse relationship between elevated delta bilirubin and lower albumin level might indicate that the chronicity of the disease may be the predominant factor in hepatobiliary obstruction or hepatocellular injury that may worsen the pathology of obstructive or hepatic jaundice.
Keywords: Delta bilirubin; Albumin; Obstructive jaundice; Hepatic jaundice; Total bilirubin
Introduction
Jaundice, a pathophysiological term used to describe an increase in the amount of bilirubin in the body [1]. However, the major causes of pathological jaundice include blood incompatibilities and diseases, hereditary syndromes, several forms of hepatitis, cirrhosis of the liver and other liver diseases, bile duct blockage along with infections and certain drug medications [2,3]. Although, liver a large complex organ that is well designed for performing a pivotal role in central metabolism, bio molecule synthesis and excretion [4]. Conjugate bilirubin, a water-soluble molecule, selectively absorbed by liver hepatocytes and still considered as one of the major decisive factors to assess the excretory function of a liver. In contrast, unconjugated bilirubin is an endogenous anion derived from haemoglobin degradation from the erythrocyte, which is a hydrophobic part of bilirubin, transported through the blood bound to serum albumin, and once it arrives in liver became conjugated with glucuronic acid by the reaction catalysed by the enzyme UDP-glucuronyl transferase [5,6]. Serum albumin, a major plasma protein is frequently employed as an indicator of the hepatocyte’s ability to carry out synthetic function of liver. However, serum albumin level does not alter in mild liver damage, but readily declines in the face of sub-massive liver necrosis [7]. Conversely, γ Glutamyl transpeptidase (GGT), a membrane bound glycoprotein enzyme, catalyses the transfer of γ glutamyl group to other peptides, and found large amounts in the kidneys, pancreas, liver and intestine. Earlier studies have suggested that GGT is frequently increased in acute hepatitis and also in cholestasis. Although, GGT does not rise with bone disease or active growth as occurs with alkaline phosphatase, and in such situation measurement of GGT serve as a useful marker in the diagnosis of cholestatic disorders [8,9].
It has been found in certain sera that contains a third bilirubin species referred to as delta-bilirubin, which forms by spontaneous transesterification of bilirubin glucuronide esters to exposed carboxyl groups on various serum proteins (mainly on albumin) among the subjects with cholestasis [10,11]. Delta-bilirubin is a nontoxic and nonenzymatic covalently bound complex with albumin, has a long half-life irrespective of hepatorenal function, which is excreted neither in urine nor in bile [12]. However, total bilirubin, direct or indirect bilirubin, albumin and GGT have already been recognized as biomarker for differentiation and pattern of haemolytic, hepatic or obstructive jaundice. They are also helpful to assess the severity of the disease and predict the outcome of certain liver disorders [13,14]. But, to the best of our knowledge, there are no ample amount of data has been evaluated to elucidate the exact association of serum delta bilirubin level and impaired excretory or synthetic function of liver in the context of obstructive, hepatic or haemolytic jaundice. Hence, the study was intended to investigate serum delta bilirubin level and impaired hepatic function across the spectrum of jaundice i.e. haemolytic, hepatic and obstructive, and to evaluate the exact relationship of delta bilirubin and total bilirubin or albumin or GGT activity in different types of jaundice.
Materials and Methods
Study subjects
In the present observational case control study, we have enrolled 50 subjects with chronic obstructive jaundice (more than three-month duration), 50 patients with hepatic jaundice (acute or chronic) and 50 individuals those who have haemolyticjaundice. Further, 50 control subjects were enrolled in this study without having any hepato biliary disorder but admitted due to routine health check-up. Age, sex and blood pressure were matched among all the enrolled study subjects. Subjects having neonatal hyperbilirubinemia, drug induced hepatitis, alcoholic or non-alcoholic fatty liver disease, hepatic carcinoma, Wilson’s disease and Gilbert syndrome were excluded from this study. Obstructive, hepatic or haemolytic jaundice were diagnosed by the documentation of patient clinical history, radiological and other lab medicine-based investigations. Patients admitted or attending the medicine or gastroenterology OPD clinic of Calcutta Medical Research Institute (CMRI), Kolkata were considered for the study. Enrolled study subjects, those who fulfilled all the inclusion and exclusion criteria were finally enrolled in this study. All the subjects enrolled in this study were belonging to same geographical area (Gangtic Delta), Eastern India. The study protocol was approved by the institute’s ethical committee. The study protocol was explained to all the considered patients and only those who gave informed written consent were finally included.
Sample collection and processing
Venous blood samples (4ml in clot vial, collected after 8 hours fast and physical rest for 30 minutes) were centrifuged at 3000rpm for 10 minutes at 4 °C temperatures to separate cellular components to collect serum from clot vial. Serum samples were collected in cryocube vials for the estimation of serum total bilirubin, direct and delta bilirubin, albumin and GGT.
Total bilirubin
Total bilirubin was measured by photometry in Vitros 250 system (Ortho-Clinical Diagnostics, Johnson & Johnson, Buckinghamshire, United Kingdom). The method uses dyphylline to separate unconjugated bilirubin from albumin. Unconjugated bilirubin, conjugated bilirubin, and albuminlinked bilirubin (delta) subsequently react with the diazonium salt 4-(N-carboxymethylsulfonyl) benzenediazonium hexafluorophosphate to produce azobilirubin chromophores that have similar molar absorptivities and absorbance maximum around 520nm. The concentration of total bilirubin is determined by measuring the azobilirubin chromophores at two wavelengths through the transparent support. The reflectance measurement at 460nm corrects for spectral interferences. The measuring range of this method is 0.1 to 27mg/dl in serum.
Delta bilirubin and bilirubin unconjugate + conjugate (Bu Bc)
We have used Vitros 250 system (Ortho-Clinical Diagnostics,
Johnson & Johnson, Buckinghamshire, United Kingdom) dry
slides to measure total bilirubin (TBil) and, bilirubin unconjugate
+ conjugate (BuBc) slides to measure delta bilirubin fraction that
bounds covalently with albumin. Finally, we have derived the
amount of delta bilirubin by using following formula.
[TBil- (Bu+Bc) = delta bilirubin]
Bu + Bc present in the sample was determined by end point
photometric assay by using dual wavelength (400 and 460nm) at
37 °C in Vitros 250 system. The measuring range of Bu and Bc is 0.0
to 27.0mg/dl in serum.
Albumin
Serum albumin was measured by Vitros 250 system by reflectance photometry. Albumin present in the sample binds with bromcresol green (BCG) dye present in the reagent slide layer. This binding result in a shift in wavelength of the reflectance maximum of the free dye. The color complex formed was measured by reflectance spectrophotometry at 630nm wavelength. The assay was performed at 37 °C.
Gamma glutamyl transpeptidase (GGT)
Serum GGT was measured by Vitros 250 system. GGT present in the sample catalyses the transfer of the gamma-glutamyl-paranitroanilide to glycyl glycine, with simultaneous production of para- nitroaniline. The rate of change in reflection density was measured at multipoint rate by using reflectance photometry at 400nm wavelength. This rate of change in reflection density was used to calculate the GGT activity present in the sample at 37 °C. The measuring range of the assay was 10 to 1400U/L.
Statistical analysis
All variables were expressed as Mean ± SD (Standard deviation). The Mean obtain from different sample groups were compared by non-parametric Mann-Whitney U ‘t’ - test. Mean obtained from two normally distributed sample group were compared by Student’s unpaired two tailed ‘t’ test. Nonparametric multiple correlations were performed to find out the relation between delta bilirubin and rest of the variables enrolled in the present study. To find out the correlation between two variables, Spearman nonparametric correlation coefficient was used. A value of p < 0.05 was considered as statistically significant. A correlation coefficient (r) value between (-1.0 to -0.5 or 1.0 to 0.5), (-0.5 to -0.3 or 0.3 to 0.5), (-0.3 to -0.1 or 0.1 to 0.3) and (-0.1 to 0.1) were considered as robust, moderate, weak and none, respectively. All statistical analysis was performed by using Graph Pad prism software (version 5, 2007, Sandiego, California, USA).
Results
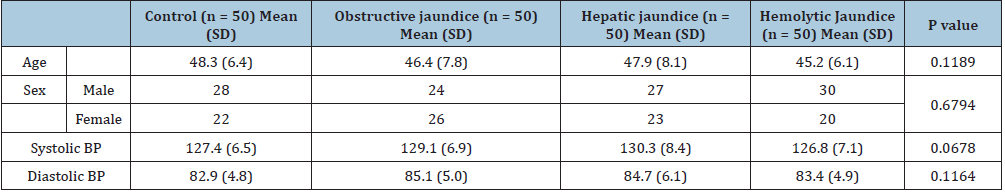
There was no significant statistical difference was found in age, sex distribution, systolic and diastolic blood pressure among all the enrolled study subjects (p > 0.05) (Table 1). A significant elevation of serum delta bilirubin was found among the subjects with chronic obstructive jaundice (0.13 ± 0.18 vs 1.66 ± 1.68; p= 0.0001), Hepatic jaundice (0.13 ± 0.18 vs 1.0 ± 0.99; p=0.0001) and the patients those who have haemolytic jaundice (0.13 ± 0.18 vs 1.11 ± 1.39; p=0.0001) compared to control individuals. Further, the subjects with chronic obstructive jaundice, showed significantly elevated delta bilirubin level even compared to the individuals having hepatic (p=0.0347) or haemolytic jaundice (p=0.0394). Whereas, no significant difference was noted in serum delta bilirubin level in between the individuals with hepatic or haemolytic jaundice (p=0.4977) (Figure 1A). Percentage of delta bilirubin was found remarkably high among the subjects with chronic obstructive jaundice (p=0.0001) and hepatic jaundice (p=0.0002) compared to control group, though no significant change was noted in between control and the subjects those who have been enrolled as haemolytic group in the present study (p=0.412). Moreover, delta bilirubin % was found strikingly high among the subjects with chronic obstructive (p=0.0006) and hepatic jaundice (p=0.047) even compared to the individuals having haemolytic jaundice (Figure 1B). A remarkable increase in serum bilirubin was found among chronic obstructive jaundice (0.65 ± 0.35 vs 5.23 ± 5.9; p=0.0001), hepatic jaundice (0.65 ± 0.35 vs 3.69 ± 3.4; p=0.0001), and haemolytic jaundice (0.65 ± 0.35 vs 3.98 ± 3.14; p= 0.0001) patients compared to control individuals. Further, an elevated trend of serum bilirubin was noticed among the subjects with chronic obstructive jaundice compared to hepatic (p=0.1961) or haemolytic group (p=0.7616) but the difference was not significant (Figure 1C).
Figure 1:Bar column of figure 1 A, B and C indicates mean (standard error) of serum delta bilirubin, delta
bilirubin % and total bilirubin level among all the enrolled study subjects respectively.
A significantly elevated serum delta bilirubin was found among the subjects with chronic obstructive jaundice,
hepatic jaundice and haemolytic jaundice group compared to control individuals. Subjects with chronic
obstructive jaundice have showed significantly elevated delta bilirubin level compared to the individuals having
hepatic or haemolytic jaundice. Percentage (%) of delta bilirubin was found remarkably high among the subjects
with chronic obstructive jaundice and hepatic jaundice compared to control group, but no significant change was
noted in between control and haemolytic group. Delta bilirubin % was found strikingly high among the subjects
with chronic obstructive and hepatic jaundice compared to haemolytic jaundice group. A remarkable increase
in serum bilirubin was found among chronic obstructive jaundice, hepatic jaundice and haemolytic jaundice
patients compared to control individuals. Further, an elevated trend of serum bilirubin was noticed among the
subjects with chronic obstructive jaundice compared to hepatic or haemolytic group, but the difference was not
significant.
Table 1: Clinical characteristics of healthy control, Obstructive jaundice, hepatic jaundice and haemolytic jaundice subjects: Table indicates the comparison of different groups enrolled in the present study shows no statistically significant difference for sex distribution, age, and Blood pressure (BP). p<0.05 was considered as minimum level of significance. N indicates sample size.
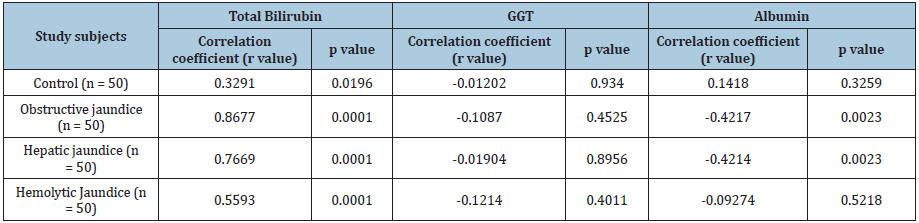
A noticeable decrease in serum albumin was noted among chronic obstructive jaundice compared to the patients having hepatic jaundice (2.85 ± 0.73 vs 3.24 ± 0.87; p=0.0177) and control individuals (2.85 ± 0.73 vs 3.94 ± 0.54; p=0.0001). Further, a significant decrease was also noted among the subjects with hepatic (3.24 ± 0.87 vs 3.94 ± 0.54; p=0.0001) and haemolytic jaundice (3.19 ± 0.85 vs 3.94 ± 0.54; p=0.0001) compared to the control group. But no significant difference was noted in serum albumin level in between the individuals with hepatic and haemolytic jaundice (p=0.7669) (Figure 2A). A significant exceptionally high GGT was found in chronic obstructive jaundice group compared to the patients having hepatic jaundice (149.4 ± 181.2 vs 636.5 ± 603.9; p=0.0001), haemolytic jaundice (24.3 ± 9.7 vs 636.5 ± 603.9; p = 0.0001) and control individuals (636.5 ± 603.9 vs 30.4 ± 19.7; p=0.0001). Moreover, the level was found elevated in hepatic jaundice subjects compared to haemolytic (p=0.0001) or control group (p=0.0001). No significant difference was noted in between haemolytic or control group (p=0.3848) (Figure 2B). Serum delta bilirubin was found in a robust significant correlation with serum total bilirubin in obstructive jaundice (r=0.8677, p=0.0001) or hepatic (r=0.7669, p=0.0001) or haemolytic jaundice (r=0.5593, p= 0.0001) group. Even more, a noticeable moderate correlation was also noted in between delta and total bilirubin among control group (r=0.3291, p=0.0196). We couldn’t find any significant relation in between elevated GGT level and delta bilirubin level among all the enrolled study subjects. Whereas, decreased serum albumin was found in a significant inverse correlation with elevated delta bilirubin level among the subjects with obstructive (r= -0.4217, p=0.0023) and hepatic jaundice (r=-0.4214, p=0.0023). However, no significant relation has been observed in between serum albumin or delta bilirubin among control (r= 0.1418, p=0.3259) or haemolytic jaundice group (r=-0.09274, p=0.5218) (Table 2).
Figure 2:Box and Whisker plot of figure 2 represents mean (+ sign), median, upper median, lower median and
minimum to maximum data range of serum albumin among all the enrolled study subjects.
Significantly decreased serum albumin was noted among chronic obstructive jaundice patients compared to the
individuals having hepatic jaundice and control subjects. Serum albumin was decreased significantly among
the subjects with hepatic and haemolytic jaundice compared to the control group. Aligned dot plot of figure 2B
represent median with range of serum GGT activity among all the study subjects. A remarkable high GGT activity
was found in chronic obstructive jaundice group compared to the patients having hepatic jaundice, haemolytic
jaundice and control individuals. Moreover, the level was found elevated in hepatic jaundice subjects compared
to haemolytic or control group. No significant difference was noted in between haemolytic or control group.
Table 2: Table indicates the value of correlation coefficient (r) obtained from the multiple correlation study in between serum delta bilirubin and three biochemical parameters, i.e. serum total bilirubin or GGT or serum albumin among different study group.
N indicates sample size.
p<0.05 was considered as minimum level of significance.
Discussion
Elevated total bilirubin along with unconjugated hyperbilirubinemia may results from a derangement of bilirubin conjugation in the hepatocyte. Extreme heme metabolism, as a result of haemolysis or reabsorption of a large hematoma increases significant unconjugated bilirubin level in haemolytic jaundice. Pattern of haemolytic jaundice depends on abnormal red blood cell life span and may occur because of red cell membrane or enzyme abnormalities. Autoimmune disorders, drugs, and hemoglobinopathies are also considered as important etiologies of haemolysis [15,16]. The predominant causes of conjugated and unconjugated hyperbilirubinemia along with elevated total bilirubin are intrahepatic cholestasis and extrahepatic obstruction of the biliary tract. Indeed, viral infection, chronic alcoholism, different autoimmune disorders are considered as principle causal factor for the occurrence of hepatic jaundice. In contrast, obstructive jaundice manifested by hyper bilirubinemia along with elevated total bilirubin level may be due to intrinsic or extrinsic obstruction of hepatobiliary duct system and that in turn leads to cholecystitis, or inflammation of the gallbladder [5,6,17,18]. In the present study significantly increased serum total bilirubin was found in chronic obstructive jaundice patients compared to the individuals those who have hepatic or haemolytic jaundice, which was in accord with pervious findings. Further, the subjects with chronic obstructive jaundice, showed significantly elevated delta bilirubin level compared to the individuals having hepatic or haemolytic jaundice, whereas the delta bilirubin % was found significantly high among chronic obstructive and hepatic jaundice patients even compared to the individuals having haemolytic jaundice. Kozaki et al [10] reported that increase percentage of serum delta-bilirubin might indicate an effective biliary drainage and assessment of serum delta-bilirubin helps to distinguish the good drainage patients from the poor drainage patients. Another study has also reported that the concentration and proportion of total and delta-bilirubin in serum reflect the duration of jaundice and helps to evaluate the efficacy of biliary drainage. Further, it has also been reported that longer duration of cholestasis results in increased delta bilirubin level among the infants with biliary atresia [12,19]. Although, we found a significant correlation between elevated level of delta bilirubin and total bilirubin among all the enrolled patient group, but the strongest correlation was noted among the subjects those who have obstructive jaundice. So, based on earlier substantial study reports and our present findings it may be said that elevated delta bilirubin might have an intimate association with elevated serum total bilirubin in certain post hepatic inflammation or in an obstruction state. Hence, measurement of elevated delta bilirubin along with total bilirubin may provide us with a prospective tool for assessing effective biliary drainage along with duration or chronicity of jaundice, which already has been found among the subjects with obstructive jaundice. Thus, elevated delta bilirubin percentage may help to differentiate the diagnosis of obstructive jaundice from hepatic or haemolytic jaundice. Serum albumin, synthesized in rough endoplasmic reticulum of healthy hepatocytes, closely reflects an effective hepatocellular function. A low level of serum albumin concentration is a late finding in liver disease and suggests the possibility of a chronic liver disease [20]. However, decreased serum albumin was detected in hepatocellular jaundice, which was found to be associated with decreased hepatocyte function due to hepatocyte necrosis in a case of viral hepatitis. Earlier studies found normal serum albumin concentrations among the patients with biliary obstruction or acute hepatitis, and that may be due to prolong half-life of albumin in plasma [around 20 days] [21,22]. In contrast, Younes et al. [23] observed hypoalbuminemia among experimental rat model of obstructive jaundice, after two weeks of bile duct obstruction and subsequent biliary decompression. Another study also reported a significantly decreased serum albumin level among the individuals with hepatic and obstructive jaundice [24]. In the present study, low level of serum albumin was detected among all the enrolled study groups compared to control, but the subjects with obstructive jaundice has shown significantly decreased value of albumin even compared to hepatic or haemolytic jaundice group. Moreover, decreased serum albumin was found in a significant inverse correlation with elevated delta bilirubin level among the subjects with obstructive and hepatic jaundice. Low serum albumin level along with elevated serum delta bilirubin might indicate the chronicity of liver disease whether it is due to hepatic or obstructive origin. Decreased hepatocellular function among the subjects with obstructive jaundice, showed by showing lower serum albumin level, may further aggravated by persistent hepatobiliary obstructions. However, decreased serum albumin among the subjects with hepatic jaundice is consistent with earlier findings, but based on our study design certainly we are not able to explore the underlying mechanism involved in low serum albumin in haemolytic jaundice. Primary and secondary hepatobiliary disorders are considered as predominant factor associated with increased serum GGT activity. A moderate range of elevation is generally found in diffuse hepatic cell injury due to toxic or infectious hepatitis. In contrasts, higher level of GGT activity has been reported in cholestasis due to chronic intrahepatic or extrahepatic biliary obstruction. Earlier studies have documented an earlier increase of GGT in cholestatic disorders, and the elevated level persists in serum longer than alkaline phosphates. In acute viral hepatitis the levels of GGT may reach highest peak in the second or third week of illness and in some cases, they remain elevated for 6 weeks [14,25-28]. In our present study, significant elevation of serum GGT activity was found among the subjects with obstructive and hepatic jaundice compared to haemolytic or control individual. But we couldn’t find any significant correlation in between elevated GGT level and delta bilirubin level among all the enrolled study subjects. However, our findings are consistent with previous reports and further support the fact that chronic hepatobiliary obstructions might be the major precipitating factor associated with increased GGT activity in serum.
Conclusion
In conclusion, it may be hypothesized that elevated delta bilirubin, GGT activity along with lower serum albumin, and significant inverse relationship between delta bilirubin and albumin level might be the key findings associated with the occurrence of obstructive and hepatic jaundice. These findings closely indicate that the chronicity of the disease might be the predominant factor in hepatobilliary obstruction or hepato cellular injury that may worsen the pathology of obstructive or hepatic jaundice. However, further studies in a larger cohort of patients are needed to establish these findings as potential tool for differential diagnosis of obstructive, hepatic or haemolytic jaundice.
Acknowledgement
Authors profoundly thank Mr. Ravi Sinha, Zonal Sales Manager,
Ortho clinical Diagnostics for his enthusiastic support and helpful
suggestions.
Authors profoundly thank Mr. Rajib Banerjee and Mr. Sugata
Misra for their technical support and helpful suggestions.
References
- Green RM, Flamm S (2002) AGA technical review of evaluation of liver chemistry tests. Gastroenterology 123(4): 1367-1384.
- Roche SP, Kobos R (2004) Jaundice in the adult patient. Am Fam Physician 69(2): 299-304.
- Dufour DR, Lott JA, Nolte FS (2000) Diagnosis and monitoring of hepatic injury i Performance characteristics of laboratory tests. Clin Chem 46(12): 2027-2049.
- Aithal GP, Rawlins MD, Day CP (2000) Clinical diagnostic scale: a useful tool in the evaluation of suspected hepatotoxic adverse drug reactions. J Hepatol 33(6): 949-952.
- Dufour DR, Lott JA, Nolte FS (2000) Diagnosis and monitoring of hepatic injury II. recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 46(12): 2050-2068.
- Berk PD, Noyer C (1994) Clinical chemistry and physiology of bilirubin. Semin Liver Dis 14(4): 346-355.
- Seidman DS, Paz I, Stevenson DK (1991) Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics 88: 828-833.
- Rosalki SB, Tarlow D, Rau D (1971) Plasma gamma-glutamyl transpeptidase elevation in patients receiving enzyme-inducing drugs. Lancet 2(7720): 376-377.
- McCullough AJ (2002) Update on nonalcoholic fatty liver disease. J Clin Gastroenterol 34(3): 255-262.
- Kozaki N, Shimizu S, Higashijima H (1998) Significance of serum delta-bilirubin in patients with obstructive jaundice. J Surg Res 79(1): 61-65.
- Harpavat S, Finegold MJ, Karpen SJ (2011) Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics 128(6): e1428-e1433.
- Wen Y, Philip R, John CM (2015) Factors Determining delta-bilirubin levels in biliary atresia infants running head: delta-bilirubin in biliary atresia. J Pediatr Gastroenterol Nutr 60(5): 659-663.
- Bonnand AM, Heathcote EJ, Lindor KD (1999) Clinical significance of serum bilirubin levels under ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. Hepatology 29(1): 34-43.
- Thapa BR (2007) Liver function test and their interpretation. Indian J Pediatr 74 (7): 663-671.
- Pashankar D, Schreiber RA (2001) Jaundice in older children and adolescents. Pediatr Rev 22(7): 219-226.
- Sackey K (1999) Hemolytic anemia. Pediatr Rev 20: 152-158.
- Schramm C, Kanzler S, Zum BKH (2001) Autoimmune hepatitis in the elderly. Am J Gastroenterol 96(5): 1587-1591.
- Kalloo AN, Kantsevoy SV (2001) Gallstones and biliary disease. Prim Care 28(3): 591-606.
- Higashijima H, Yamashita H, Makino I (1996) Significance of serum delta bilirubin during obstructive jaundice in dogs. J Surg Res 66(2): 119-124.
- Feld JJ, Heathcote EJ (2003) Epidemiology of autoimmune liver disease. J Gastroenterol Hepatol 18(10): 1118-1128.
- Doumas BT, Peters T (1997) Serum and urine albumin: A progress report on their measurement and clinical significance. Clin Chim Acta 258(1): 3-20.
- Dufour DR, Lott JA, Nolte FS (2000) Diagnosis and monitoring of hepatic injury II recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 46(12): 2050-2068.
- Younes RN, Vydelingum NA, Derooij P (1991) Metabolic alterations in obstructive jaundice: effect of duration of jaundice and bile-duct decompression. HPB Surgery 5(1): 35-48.
- Sunanda V, Ramesh M, Sangeeta S (2012) Study of biochemical markers in jaundice: our experience. Int J Biol Med Res 3(1): 1365-1368.
- Giannini E, Botta F, Fasoli A (2001) Increased levels of gamma GT suggest the presence of bile duct lesions in patients with chronic hepatitis C: absence of influence of HCV genotype, HCV-RNA serum levels, and HGV infection on this histological damage. Dig Dis Sci 46(3): 524-529.
- McCullough AJ (2002) Update on nonalcoholic fatty liver disease. J Clin Gastroenterol 34(3): 255-262.
- Maggiore G, Bernard O, Hadchouel M (1991) Diagnostic value of serum gamma-glutamyl transpeptidase activity in liver diseases in children. J Pediatr Gastroenterol Nutr 12: 21-26.
- Jansen PLM, Muller M (2000) The molecular genetics of familial intrahepatic cholestasis. Gut 47(1): 1-5.




No comments:
Post a Comment