Exposure to Secondhand Smoke Exacerbated
Natural Aging Cardiac Hypertrophy by Jia-Ping Wu in Open Journal of Cardiology & Heart Diseases_American Journal of Cardiology
Abstract
Secondhand Smoke (SHS) exposure is associated with an increased risk of coronary artery disease.
This study’s aim was to investigate the relationship of SHS exposure in old rats’ left ventricle impaired
and fibrosis. To explore the mechanism of cardiac remodeling of exposure to SHS exposure whether it
was exacerbated cardiac impaired, especially in the older left ventricle. The animals were placed in a
transparent exposure chamber, connected to smoking device and exposed to 15 cigarettes, smoke for
30 min, twice a day, 6 days/week, for 1 month. Histopathologic of left ventricular sections were stained
with hematoxylin-eosin staining (H&E) and Masson’s trichrome. Left Ventricular (LV) morphological
variables assessed using H&E stained and Mass weight changes. The cardiac structures were measured
by echocardiographic analysis.
LV remodeling and fibrosis-related proteins were detected by gelatin zymography and western blotting
analysis. Inflammatory and hypertrophy related proteins were also detected. Results showed in old
rats’ group and old rats in the secondhand smoke exposure group (Old SHS Exp) were observed LV
wall and mass increased, collagen accumulation and fibrosis, and extracellular space increased. From
echocardiographic results, we found LV functions were apparently decreased, LV interventricular septum
at systolic and diastolic diameters increased in the Old SHS Exp group. Cardiomyocyte width was increased
in old rats, but the length was increased in Old SHS Exp group. Reduced MMP 2 proteins expression and
TIMPs increased were induced fibrosis in the Old SHS Exp group. JNK1, p38, IL-6, TNFα were increased
by western blotting and immunohistochemistry antibody-positive expression was observed in the Old
SHS Exp group.
Keywords: Secondhand smoke exposure; Left ventricle; Cardiac impaired; Left ventricular hypertrophy;
Echocardiographic; Cardiomyocyte
Introduction
Secondhand smoke (SHS) exposure is a serious health hazard causing the risk of
coronary heart diseases. It causes a wide range of damaging health effects in children and
older. SHS exposure affected on the cardiovascular system, including atherosclerosis, arterial
stiffness and coronary cardiac disease [1]. In the previous studies reported, SHS exposure
is the combination of smoke given off by the burred end of a tobacco or cigarette product
to exposure to environment and the smoke exhaled by the smoker [2]. However, low-level
chronic cigarette smoke exposure harmful older adults are still unclear. In this study, we use
the older rats who underwent 15 cigarette exposure 30 minutes to determined left ventricular
remodeling and function. Hypertrophy is an initial adaptive response. Long-term exposure to
secondhand smoke increases the risk of developing cancer in younger and elderly people [3].
Low levels of fine particulate exposure from secondhand cigarette smoke are sufficient
to induce increase the risk of cardiovascular disease mortality. The SHS exposure-response
relationship between cardiovascular disease mortality and fine particulate matter is relatively
steep at low levels of SHS exposure and flattens at higher exposures [4]. There are many
compensatory mechanisms to increase cardiac workload and stimulation of left ventricular
sustains. However, aging is a progressive disease which is a typic natural course whose
worsening of the disease until death occurs. Slowly progressive age-related diseases are also
chronic diseases; many are also degenerative diseases [5].
The aging mature organism that occurs normally the gradual changes in the structure
over time and increases the probability of death. This growing process is unavoidable.
These physiologic changes of old cardiac include left ventricular
hypertrophy, increased cardiac fibrosis, and valvular degeneration.
Cardiovascular disease is a major risk factor for the aging cause of
death [6]. Aging changes in the elderly heart are associated with
physiological Left Ventricular Hypertrophy (LVH). However, SHS
exposure is associated with pathological LVH [7]. SHS exposure in
the elderly maybe leads to cardiovascular diseases such as heart
failure and atherosclerosis. SHS exposure in the old heart is still
unclear. Heart failure is a related change in cardiac morphology,
including decreased in myocyte number, increased in myocytes
size decreased in matrix connective tissue, increased in left
ventricular wall thickness increased in conduction fiber density
and decreased in sinus node cell number [8-10]. SHS exposure in
the elderly may stimuli first induce a phase of cardiac hypertrophy,
especially in left ventricles individual. Health aging changes may
produce clinical heart disease and may mimic heart diseases, such
as cardiomyopathy, aortic valve calcium and mitral valve annular
calcium [11,12]. Therefore, we detected the molecular mechanisms
behind the aging in SHS exposure treatment to identify pathological
of cardiac disease disorder and elusive.
Materials and Methods
Animals
We purchased SD rats of 6 weeks years-old age from National
Science Council Animal Center and used according to the guidelines
of the Helsinki Declaration. One group of rats of 6-weeks-old rats
as our young, another group of rats of 18-months-old as our older
age groups. Rats were housed in cages in an environmentally
controlled animal room. Use committee approved animal care and
experiments. Animal room temperature is maintained at 25°C, and
relative humidity was approximately 40%.
Secondhand Smoke (SHS) exposure experimental
The elderly SD rats placed in a whole-body transparent
exposure chamber with a volume of approximately 95x85x85cm,
connected to a smoking device. Filtered air is introduced into the
chamber at a low rate. Puffs of SHS exposure were collected in the
smoking chamber, is then thrown into the chamber for 30 minutes.
The smoke is released at a rate of 15 cigarettes, twice a day in the
morning and twice in the afternoon with 30 minutes rest intervals,
until the end of 4 weeks.
Echocardiography
After 4 weeks of exposure treatment, all the rats underwent
echocardiographic study according to the previously described
method. Rats used anesthetized with ketamine hydrochloride
(50mg/kg) and xylazine hydrochloride (1mg/kg). Transthoracic
echocardiography was performed at 4 weeks after secondhand
smoke (SHS) exposure using a Hewlett-Packard Sonos 5500
ultrasound machine with a 7.5-15 MHz linear-array transducer,
as described previously. In the short-and long- axis parasternal
view, we could obtain a transverse left ventricular one-dimensional
image, the ultrasound beam right below the mitral valve plane
between the papillary muscles by using the 2D image as a guide for
positioning. The M-mode image was recorded and analyzed offline.
Hematoxylin-eosin (H&E) and massons trichrome (mt)
stained
Left ventricular cross-sections were cut 10μm thick and placed
on slides. Slides deparaffinization and dehydration were performed.
They were passed through a series of graded alcohols from 100% to
90% to 70%, 5min each. Hematoxylin-eosin and Masson trichome
stained were prepared, incubated for 5min at room temperature.
After rinsing with Phosphate-Buffered Saline (PBS), each slide was
then soaked with 85% alcohol, 100% alcohol for 5min. After rinsing
with water, each slide was then soaked with 85% alcohol, 100%
alcohol for 15 min. Stained sections were then rinsed with PBS and
air-dried before mounting.
Gelatin zymography
Proteins were separated by 8% non-reducing SDS-PAGE
copolymerized with 1mg/ml gelatin. The PAGE was washed at
room temperature twice 10 minutes with 2.5% Triton-X 100 and
subsequently incubated overnight at 37℃ for maximum sensitivity
in Zymogram Developing Buffer mixture (50mmol/L Tris-HCl,
pH 7.4 containing 5mmol/L CaCl2 and 1μmol/L ZnCl2). Gels were
stained with Coomassie brilliant blue G250 (Methanol, Acetic acid,
and water mix) and then destained. The amounts of proenzyme and
active metalloproteinase were analyzed by densitometry scanning
of the gel.
Western blot
We prepared the tissue extract samples as described above.
SDS-PAGE was carried out with polyacrylamide gels. The samples
were electrophoresed at 100V for 1hr. Electrophoresed proteins
were transferred to PVDF membranes at 150mA for 2hr. We
incubated PVDF membranes in blocking buffer (5% non-fat milk
in PBS-Tween) for 1hr at room temperature. Polyclonal antibodies
against JNK1/2, p38α, IL-6, TNFα, MMP2, MMP9, TIMP-1, TIMP-2,
TIMP-3 and TIMP-4 (Santa Cruz, Dallas, Texas, U.S.A.) were diluted
1:200 in antibody buffer (TBS). Incubations were performed at
room temperature for 3.5hr. We washed the immunoblots three
times in 5ml PBS-Tween for 10min and then immersed in the
second antibody solution containing alkaline phosphatase goat
anti-rabbit IgG for 1hr and diluted 1,000-fold in binding buffer.
Color development was presented in ECL chemiluminescence.
Ethical Statement
Animals guidelines for the animal experimental use of Taipei
Medical University Animal Care and Use Committee (IACUC) (LAC-
2019-0264) and ARRIVE Guidelines were followed. The Taiwan
Council approved the animal care and experiment. All procedures
followed have been performed in accordance with the ethical
standards laid down in the 1964 Declaration of Helsinki and its
later amendments.
Statistical Analysis
Quantitation was carried out by scanning and analyzing the
intensity of the hybridization signals using the FUJIFILM Imagine
program for western blot analysis. Statistical analysis of the data
was performed using Sigma Stat software. Results were expressed as mean ±
SEM. Statistical analysis was performed using the analysis
of variance. When assessing multiple groups, one-way ANOVA was
utilized with the students t-test was used when indicated.
Result
Histopathologic of a left ventricular cross-sectional analysis
assessed cardiac changes in old rats in cigarette smoke exposure
by H&E stained and masson’s stained. To investigate the effects
of secondhand smoke (SHS) exposure on cardiac functions and
structural changes were determined in rats model recommended
for gerontological. Heart cross-sections were stained with Massonss
trichrome or hematoxylin/eosin staining for visualization of
morphology and identification of the location. A cross-sectional
analysis assessed left ventricular changes in old rats and old rats
in the SHS exposure group. As shown in Figure 1A. left ventricular
chamber becomes narrowed in old rats in the SHS exposure group
(Old SHS Exp).
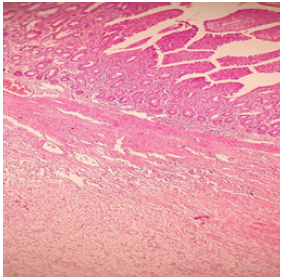
Figure 1:Representative histopathological analysis of left ventricular cross-sections with hematoxylin & eosin
(H&E) and Masson’s trichrome staining in young, old rats and old rats in the SHS exposure groups (Old SHS
Exp).
(A) Representative by hematoxylin & eosin staining of left ventricular sections in young, old and old SHS Exp
groups
(B) Representative collagen accumulation in the left ventricle by hematoxylin & eosin staining in young, old and
old SHS Exp groups. Scale bars 20μm. The images of left ventricular architectures were magnified 200x. Yellow
arrows express
(C) Representative collagen accumulation in the left ventricle by Masson’s trichrome staining in young, old and
old SHS Exp groups. Scale bars 20μm. The images of left ventricular architectures were magnified 400x. Yellow
arrows express.
At the same time, SHS exposure resulted in old rats’ left
ventricular papillary muscle deteriorated which led to left
ventricular dysfunction. Left ventricular muscle fibers interstitial
and extracellular space was broad. Muscle fibers’ rearrangement is
disordered. In old rats and old rats in the SHS exposure group, we
also could observe ECM degradation resulted in collagen release in
cardiomyocytes interstitial (Figure 1B) and collagen accumulation
induced fibrosis. Indeed, from Masson’s trichrome stained results,
we could observe blue color staining in cross-sections (Figure 1C).
Changes in structures development of heart in old rats
and old rats in SHS exposure group
Figure 2 presents heart and Left Ventricular (LV) characteristics
in young, old and old rats in the SHS exposure (Old SHS Exp). The
whole heart weights of old rats and old rats in the SHS exposure
were heavier than young rats. Aging and SHS exposure rats were
also enhanced left ventricular weights (*p<0.05 vs. young rats;
#p<0.05 vs. old rats) (Figure 2A). The HW-to-body weight and LVto-
body weight ratios were significantly increased compared with
younger age group. Compared with old rats, the HW-to-body weight
and LV-to-body weight ratios also had significantly increased
(Figure 2B) in Old SHS Exp groups (*p<0.05 vs. young rats; #p<0.05
vs. old rats). However, body weight is easily caused by the gradual
increase in a consequence of aging. Sometimes affected by alcohol,
smoke or toxic material effects. The use of tibia length has been
evidenced as more reliable than body weight. Because of in which
body weight differences may occur condition errors.
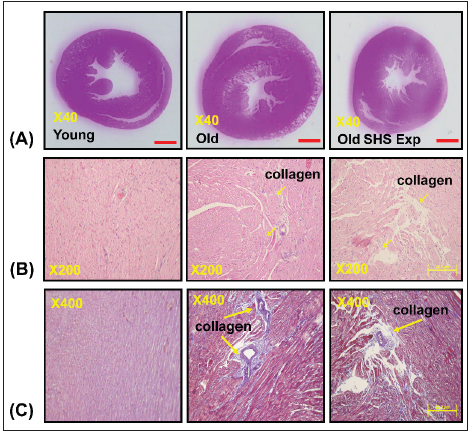
Figure 2: Left ventricular hypertrophy takes place in old rats and old rats in the SHS exposure group (Old SHS
Exp).
(A) Quantification of heart weight and left ventricle weight statistical analysis. All data are represented as the
means ± SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats
(B) Quantification of the heart weight to body ratio and the ratio of left ventricular weight to body weight statistical
analysis. All data are represented as the means ± SEM. *p<0.05 compared with young rats. #p<0.05 compared
with old rats
(C) Quantification of heart weight to tibial ratio and the ratio of left ventricular weight to tibial statistical analysis.
All data are represented as the means ±SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats
(D) Quantification of percent of area of left ventricle occupied by collage. All data are represented as the means ±
SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats
(E) Quantification of percent (%) left ventricle extramyocyte connective tissue space (area). All data are represented
as the means ±SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats (F). Quantification of
average number of myocytes per 400μm. Values were calculated from myocardial regions. All data are represented
as the means ± SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats.
Furthermore, we detected and measured HW-to-tibial and LVto-
tibial ratios to determine heart or left ventricular hypertrophy in
old rats and to exposed to SHS exposure (Old SHS Exp). The result
showed both of HW-to-tibial and LV-to-tibial ratios were increased
significantly as age increased, although exposure to SHS exposure
(Figure 3C) (*p<0.05 vs. young rats; #p<0.05 vs. old rats). Therefore,
we obtained HW-to-tibial and LV-to-tibial ratios were increased
compared with old rats. To detect whether SHS exposure led left
ventricular fibrosis exacerbated, we independently calculated
the percentage of the per cross-sectional area from hematoxylinstained
sections. Quantification of the percentage of the area of left
ventricle occupied by collagen (%), collagen area was measured
in old rats and old rats in SHS exposure group (Old SHS Exp). The
percentage of tissue attributed to collagen distribution increased
more rapidly in old rats in the SHS exposure group than in old or
young rats (*p<0.05 vs. young rats; #p<0.05 vs. old rats) (Figure 3).
To detect whether SHS exposure led left ventricular hypertrophy
exacerbated, we independently calculated the percentage of
monocytes per cross-sectional area from hematoxylin-stained
sections.
Quantification of left ventricular muscle fibers interstitial
width of extracellular space, we found left ventricular muscle
fibers interstitial become broad resulted in the percentage of
extracellular space (%) increased (*p<0.05 vs. young rats; #p<0.05
vs. old rats) (Figure 3). During old age or exposure to SHS exposure,
left ventricles from old rats and old rats in the SHS Exp group
exhibited fewer percentage per unit area than young rats (*p<0.05
vs. young rats; #p<0.05 vs. old rats) (Figure 3). But cardiomyocytes
density is increased per unit area than young rats (result does not show
in this article). Indeed, according to tissue architecture using
H&E staining analysis, we determined cell size measurement. We
observed left ventricular cell size increased width in old rats.
Left ventricular function and structures development of
left ventricle on echocardiographic analysis
The echocardiographic analysis is a primary imaging method
in the assessment of cardiac impaired and function declined
(Figure 3). Parasternal long-axis and short-axis echocardiographic
views in young, old and old rats in the SHS exposure (Old SHS
Exp) groups showing severe left ventricular hypertrophy. We
found left ventricular wall thickness increased. However, M-mode
echocardiograms result taken proximal to the papillary muscle
deterioration in old rats and old rats in the SHS exposure group
(Figure 3A). On the other hand, we found left ventricular wall
thickness increases in old rats and old rats in the SHS exposure at
systolic and diastolic. Quantification of myocardial hypertrophy for
old rats and old rats in the SHS exposure groups are displayed in
Figure 3B. Echocardiography of interventricular septal in systolic
(IVSs), interventricular septal in diastolic (IVSd), left ventricular
internal dimension at end-systolic (LVIDs), left ventricular internal
dimension at end-diastolic (LVIDd), left ventricular posterior wall
thickness in systolic (LVPWs) and left ventricular posterior wall
thickness in diastolic (LVPWd) were presented in young, old and
old rats in the SHS exposure group.
Figure 3: Histopathologic of left ventricular hypertrophy using M-mode echocardiograms in young, old rats and
old rats in the SHS exposure group (Old SHS Exp)

(A) Representative M-mode echocardiograms taken proximal from young, old and old SHS Exp group. These
images were obtained from short-and long-axis imaging at the mid-papillary level. Parasternal short-axis
echocardiography views (up-panel), parasternal long-axis echocardiography views (down-panel)
(B) Quantification of left ventricular posterior wall thickness at diastolic and systolic, interventricular septal
in systolic (IVSs), interventricular septal in diastolic (IVSd), left ventricular internal dimension at end systolic
(LVIDs), left ventricular internal dimension at end diastolic (LVIDd), left ventricular posterior wall thickness in
systolic (LVPWs) and left ventricular posterior wall thickness in diastolic (LVPWd) shown in right panel. All data
are represented as the means ± SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats
(C) Quantification of the percentage of fractional shorting and ejection fraction at diastolic and systolic. All data
are represented as the means ± SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats.
The morphological variables obtained from the
echocardiographic study are shown in Figure 3. The old rats in
SHS exposure had greater IVSs, IVSd, LVIDs, LVIDd, LVPWs and
LVPWd dimension compared with young rats (*p<0.05 vs. young
rats; #p<0.05 vs. old rats). After exposure to SHS, the old rats had
statistically greater dimensions than nonsmoking old rats did. This
variable change was used to confirm the efficacy of the exposure
of old rats to secondhand smoke (SHS). In addition, considering
the left ventricular variables, the ejection and shortening fractions
were significantly declined. As Figure 3C shown, shortening (FS%)
and ejection fraction (EF%) displayed a progressive impairment
in old rats and old rats in the SHS exposure group (Old SHS Exp)
(*p<0.05 vs. young rats; #p<0.05 vs. old rats).
Cardiomyocytes width and length of left ventricular
hypertrophy in old rats and old rats in the SHS exposure
group
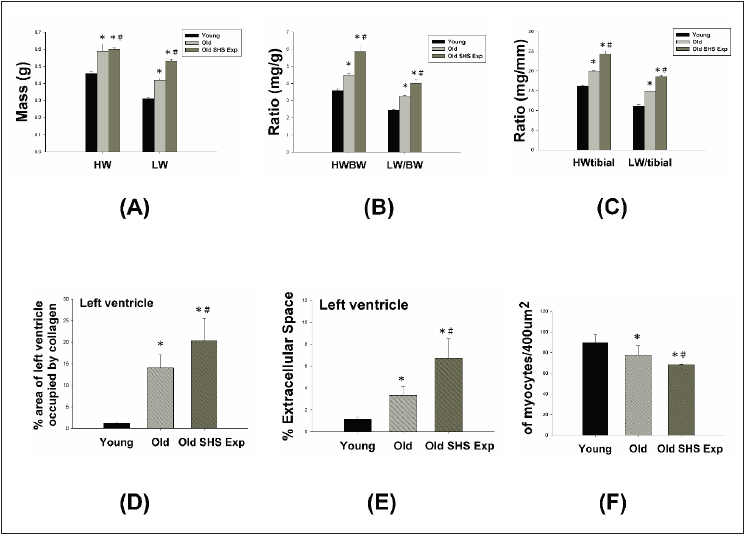
The phase of left ventricular hypertrophy during adaptive stress
or overload is individual left ventricular myocyte grown in length
and/or width as compensated or dilation hypertrophy (Figure 4A).
According to the left ventricular cell width size, old rats in the SHS
exposure compared with old rats were increased (*p<0.05 vs. young
rats). According to compared with left ventricular cell length size
in old rats and old rats in the SHS exposure group was increased
(Figure 4B). Compared with old rats, left ventricular cell length size
in old rats in the SHS exposure group was significantly increased
(*p<0.05 vs. young rats; #p<0.05 vs. old rats).
Figure 4: Longer cardiomyocytes occur left ventricular hypertrophy in the SHS exposure group by hematoxylin
& eosin stained.
(A) Representative long cardiomyocytes occur left ventricular hypertrophy using histological sections by H&E
stained to determine cell width (μm) and cell length (μm) in young, old rats and old rats in the SHS exposure
group (Old SHS Exp)
(B) Quantification of cell width (μm) and cell length (μm) in young, old rats and old rats in the SHS exposure
group (Old SHS Exp). Values are represented as the means ± SEM. *p<0.05 compared with young rats. #p<0.05
compared with old rats.
Changes to MMPs’ protein expression can explain agerelated
heart failure disease
Fibrosis occurs from changes in the balance between synthesis
and degradation of extracellular matrix components. Therefore,
we sought to determine whether aging and SHS exposure-related
collagen accumulation and fibrosis could be related to changes in
the regulation of MMP2 and MMP9. Gelatin zymography detected 2
major gelatinolytic bands, MMP-2 and MMP-9, in the left ventricular
extracts. MMP-9 gelatinolytic bands did not observe. As Figure 5A
shown. Because its regulation was the sum of pro-MMP-9 and TIMP-
1, it may be MMP-9 complexed with TIMP-1. The down regulation
of MMP-2 in old rats and old rats in the SHS exposure group was
statistically significant compared with young rats. The extent of
changes in MMP-2 gelatinolytic activity was lower than old rats
(*p<0.05 vs. young rats; #p<0.05 vs. old rats). Consistent with the
results of gelatin zymography, MMP-2 and MMP-9 protein content
as measured by western blotting was also significantly reduced
in old rats and old rats in the SHS exposure group (Old SHS Exp)
(Figure 5B).
These results suggest that MMP2/MMP9 contributed to the
remodeling of the extracellular matrix in left ventricular fibrosis.
While aging, we found MMP2 and MMP9 protein expression
decreased. Once exposure to SHS exposure, MMP2, and MMP9
protein expression were significantly lower than old rats (*p<0.05
vs. young rats; #p<0.05 vs. old rats). The dysregulation of MMP2/
MMP9 was now believed to contribute to fibrosis in old and SHS
exposure. Elevated TIMPs expression induced fibrosis is present
in old rats and old rats in SHS exposure. MMPs catalyze ECM
degradation, TIMPs is physiological inhibitors which controlled
MMPs activity.
Figure 5: Molecular mechanisms of the imbalance of MMPs and TIMPs induced ECM remodeling.
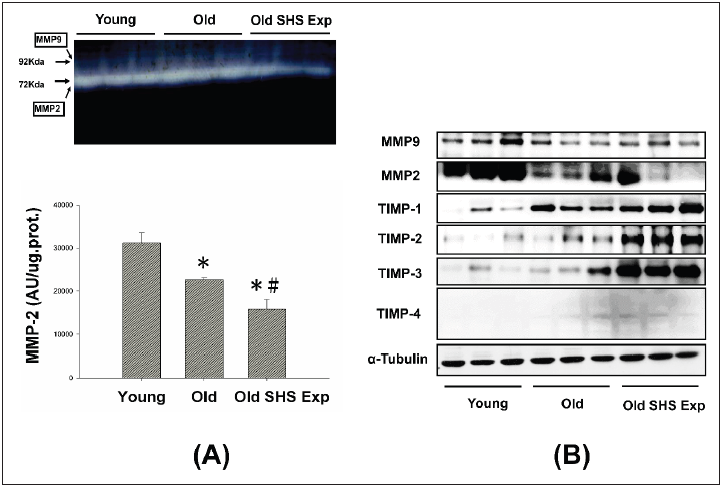
(A) Representative zymographical analysis from young, old rats and old rats in the SHS exposure group (Old SHS
Exp). Up-panel shows a gelatin zymography graphic representative of MMP-2 and MMP-9 activity. Down-panel
shows MMP-2 activity quantified by densitometry and expressed as mean pixel density. Values are represented
as the means ±SEM. *p<0.05 compared with young rats. #p<0.05 compared with old rats
(B) Protein expression activity dysregulation of MMP-2, MMP-9 and protein expression elevated of TIMP-1, TIMP-
2, TIMP-3 and TIMP-4 in old rats and old rats in the SHS exposure group (Old SHS Exp) by western blotting.
To explore whether there are regulation changes in the
expression of TIMPs, we assessed the protein expression of TIMPs
(TIMP-1, -2, -3 and -4) by Western blot. An up regulation of TIMPs
is associated with heart failure and fibrosis. To determine whether
aging or SHS exposure modulates cardiac matrix remodeling. As
Figure 5B shown, we found TIMP-1, TIMP-2, TIMP-3 and TIMP-
4 induced left ventricular fibrosis, protein expression levels
by western blotting analysis in old rats and old rats in the SHS
exposure group were significantly higher than young rats. Thus,
this apparent cause of fibrosis and heart failure can be explained by
differential regulation between MMPs and TIMPs.
SHS exposure results in higher sensitivity to
inflammation and heart failure in old rats
SHS exposure is an environmental stressor induced pathological
LVH. In this study, we determine whether low concentration SHS
exposure to old rats also will be induced pathological LVH. Results
showed p-MEK5/MEK5 and p-ERK5/ERK5 increased in old rats in
the SHS exposure group (Old SHS Exp), but not in the old hearts
(Figure 6A). To further determine the potential of inflammation in
the old hearts, we examined JNK1/2, p38α, IL-6, and TNFα protein
expression levels by western blotting and immunohistochemistry.
As Figure 6B and Figure 6C shown, JNK1/2, p38α, IL-6, and TNFα
protein expression levels were increased in old rats and old rats
in the SHS exposure group (Old SHS Exp). Immunohistochemical
study showed that densities of both old rats and old rats in the SHS
exposure hearts were higher in young rats (Figure 6C). IL-6 and
TNFα play an important role in promoting LVH and inflammation.
With greater age comes, we found that MAPK (JNK1/2 and p38)
protein expression were increased in old rats and rats in the SHS
exposure group. SHS exposure may enhance proinflammatory
cytokines (IL-6 and TNFα) and MAPK cascade expression in old
rat hearts. Thus, cytokines in left ventricular hypertrophy tissues
increased markedly in keeping with a denser inflammatory cell
infiltration.
Discussion
Secondhand Smoke (SHS) exposure has been linked to a
number of harmful health outcomes and is an important cause of
morbidity and mortality. SHS exposure is an important cause of
morbidity and mortality. There is a lot of evidence indicated that
SHS exposure a formidable health hazard. However, there is no
evidence indicated that SHS exposure presents a challenging health
hazard [13]. It is also well understood that toxic air contamination
causes lung cancer and cardiovascular diseases. In this study, we
investigated the effects of SHS exposure associated with the elderly
age, specifically in the left ventricles of male rats. As is well known,
old age in humans always accompanies an increased incidence of
atherosclerotic vascular disease and cardiovascular disease [14]. In
contrast, aging is a physiological process due to increasing injuries
and vulnerability, which reduces the ability of organisms to survive.
Aging affects various aspects of left ventricular morphology and
function and has recently been considered to be a major risk
factor for cardiovascular disease and to have effects on various
aspects of left vascular morphology and function. Aging can refer
to a time-related process, however, it is commonly used for postmaturational
processes. The main characteristics associated with
aging is a progressive decrease in physiological capacities [15].
Figure 6: The effects of SHS exposure on the activation of molecular mechanisms of inflammation and left
ventricular hypertrophy during aging.
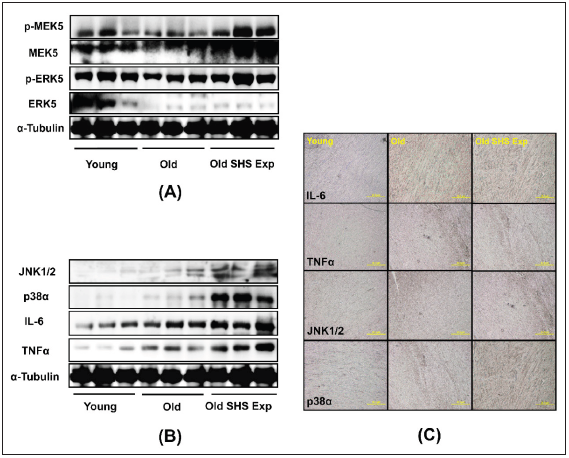
(A) Upregulation of left ventricular hypertrophy proteins, p-MEK5/MEK5 and p-ERK5/ERK5 in old rats in the
SHS exposure group by western blot
(B) Upregulation of the expression of inflammatory mediators, cytokines (IL-6 and TNFα) and MAPKs (JNK1/2
and p38), which lead to inflammation response
(C) Immunohistochemistry of IL-6, TNFα, JNK and p38 in young, old rats and old rats in SHS exposure groups.
The overall effect is highly debatable aging and disease. As
age increases, whether there will occur diseases itself. However,
the most obvious evidence of changes in the aging heart and liver.
Biological phenomena appear related to the aging process [16].
Aging exacerbates cardiac damage, leading to cardiac hypertrophy,
fibrosis [17] and dysfunction, developed compensatory
concentric hypertrophy [18] and fibrosis in response to induced
cardiomyocytes hypertrophy in a similar manner [19]. In the aging
heart, demonstrated severe left ventricular chamber dilation, wall
thinning and
fibrosis, leading to congestive heart failure. In this study, we
want to know low-level chronic cigarette smoke exposure whether
is harmful to left ventricular function in old rats and to explore the
related mechanisms. We found that changes associated with SHS
exposure lead to cardiovascular pathological outcomes resulted
in age-related disease exacerbated. We observed left ventricular
chamber narrowing and rupture and increased left ventricular wall
thickness. These results demonstrated left ventricular hypertrophy
in old rats and old rats in the SHS exposed group (Old SHS Exp).
On the other hand, we could from echocardiography results to
determine left ventricular dimension, posterior wall thickness,
interventricular septal at end-systole and end-diastole were
increased, and left ventricular function declined. Stiffening of
these fibers cause left ventricular fibrosis and could also affect the
efficient functioning. SHS exposure is linked to a number of harmful
health outcomes.
As is well known, SHS exposure is a key risk factor for pathological
hypertrophy associated with various cardiovascular disease risk
factors [20], however, the old annual human always accompanies
with atherosclerotic vascular disease and cardiovascular disease.
As the heart reaches senescence, it undergoes a modest degree
of Heart failure [21]. It is now determined the differences several
signaling molecules play unique role in regulation of old rats in the
SHS exposure group. We discuss molecular signaling mechanisms
associated with old rats in the SHS exposure, including MMPs,
TIMPs, JNK1/2, p38α, IL-6 and TNFα signaling. We suggested that
upregulation of pro-inflammatory related protein expression of
JNK1/2, p38α, IL-6 and TNFα enhanced left ventricular pathological
hypertrophy. Down-regulation of MMP2 and MMP9 in old rats in the
SHS exposure accelerated TIMPs-induced cardiac fibrosis. Despite
the evidences that chronic exposure to SHS exposure resulted in
cardiac changes [22,23], the exact mechanisms involved in lowlevel
concentration process remain to be elucidated. These explore
knowledge may influence therapeutic strategies for the treatment
of cardiovascular disease in old age.
Acknowledgment
The study was approved by National Taipei University of
Nursing and Health Sciences. No extra-institutional funding must
be reported for this article.
References
- Spychala
MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, et al. (2018)
Age-related changes in the gut microbiota influence systemic
inflammation and stroke outcome. Ann Neurol 84(1): 23-36.
- Stout
MB, Justice JN, Nicklas BJ, Kirkland JL (2017) Physiological aging:
Links among adipose tissue dysfunction, diabetes, and frailty.
Physiology 32(1): 9-19.
- Liu
P, Xu B, Cavalieri TA, Hock CE (2002) Age-related difference in
myocardial function and inflammation in a rat model of myocardial
ischemia-reperfusion. Cardiovasc Res 56(3): 443-453.
- Zhu
J, Rebecchi MJ, Glass PS, Brink PR, Liu L, et al. (2011) Cardio
protection of the aged rat heart by GSK-3beta inhibitor is attenuated:
Age-related changes in mitochondrial permeability transition pore
modulation. Am J Physiol Heart Circ Physiol 300(3): H922-H930.
- Jiang
Z, Rebecchi MJ, Qiang W, Glass PSA (2013) Chronic tempol treatment
restores pharmacological preconditioning in the senescent rat heart. Am J
Physiol Heart Circ Physiol 304(5): H649-H659.
- Dinas
PC, Koutedakis Y, Flouris AD (2013) Effects of active and passive
tobacco cigarette smoking on heart rate variability. International
Journal of Cardiology 163(2): 109-115.
- Flouris
AD, Vardavas CI, Metsios GS, Tsatsakis AM, Koutedakis Y, et al. (2010)
Biological evidence for the acute health effects of secondhand smoke
exposure. Am J Physiol Lung Cell Mol Physiol 298(1): L3-L12.
- Brink
TC, Demetrius L, Lehrach H, Adjaye J (2009) Age-related transcriptional
changes in gene expression in different organs of mice support the
metabolic stability theory of aging. Biogerontology 10(5): 549-564.
- Garvin
AM, Jackson MA, Korzick DH (2018) Inhibition of programmed necrosis
limits infarct size through altered mitochondrial and immune responses
in the aged female rat heart. Am J Physiol Heart Circ Physiol 315(5):
H1434-H1442.
- Liu
P, Xu B, Cavalieri TA, Hock CE (2002) Age-related difference in
myocardial function and inflammation in a rat model of myocardial
ischemia-reperfusion. Cardiovasc Res 56(3): 443-453.
- Juonala
M, Magnussen CG, Venn A, Gall S, Kähönen M, et al. (2012) Parental
smoking in childhood and brachial artery flow-mediated dilatation in
young adults: The cardiovascular risk in young finns study and the
childhood determinants of adult health study. Arterioscler Thromb Vasc
Biol 32(4): 1024-1031.
- Kizaki
K, Momozaki M, Akatsuka K, Fujimori Y, Uchide T, et al. (2004) Impaired
gene expression of beta 1-adrenergic receptor, but not stimulatory
G-protein Gs alpha, in rat ventricular myocardium treated with
isoproterenol. Biol Pharm Bull 27(7): 1130-1132.
- Kelly
G (2010) A review of the sirtuin system, its clinical implications, and
the potential role of dietary activators like resveratrol: Part 1.
Altern Med Rev 15(3): 245-263.
- Bard
RL, Dvonch JT, Kaciroti N, Lustig SA, Brook RD, et al. (2010) Is acute
high-dose secondhand smoke exposure always harmful to microvascular
function in healthy adults? Prev Cardiol 13(4): 175-189.
- Zhu
L, Di PY, Wu R, Pinkerton KE, Chen Y, et al. (2015) Repression of CC16
by cigarette smoke (CS) exposure. PLoS One 10(1): e0116159.
- Koutros
S, Silverman DT, Alavanja MCR, Andreotti G, Lerro CC, et al. (2016)
Occupational exposure to pesticides and bladder cancer risk. Int J
Epidemiol 45(3): 792-805.
- Yang
Z, Ming XF (2012) mTOR signalling: The molecular interface connecting
metabolic stress, aging and cardiovascular diseases. Obesity Reviews 13:
58-68.
- de
Almeida AJPO, Ribeiro TP, de Medeiros IA (2017) Aging: Molecular
pathways and implications on the cardiovascular system. Oxid Med Cell
Longev p. 7941563.
- Venn
A, Britton J (2007) Exposure to secondhand smoke and biomarkers of
cardiovascular disease risk in never-smoking adults. Circulation 115(8):
990-995.
- Sheydina A, Riordon DR, Boheler KR (2011) Molecular mechanisms of cardiomyocyte aging. Clin Sci 121(8): 315-329.
- Ungvari
Z, Csiszar A (2012) The emerging role of IGF-1 deficiency in
cardiovascular aging: Recent advances. Gerontol A Biol Sci Med Sci
67(6): 599-610.
- Gielen
S, Sandri M, Kozarez I, Kratzsch J, Teupser D, et al. (2012) Exercise
training attenuates MuRF-1 expression in the skeletal muscle of patients
with chronic heart failure independent of age: The randomized leipzig
exercise intervention in chronic heart failure and aging catabolism
study. Circulation 125(22): 2716-2727.
- Alagiyawanna
AMAAP, Veerasingam EQ, Townsend N (2018) Prevalence and correlates of
exposure to secondhand smoke (SHS) among 14 to 15-year-old
schoolchildren in a medical officer of health area in Sri Lanka. BMC
Public Health 18(1): 1240.
For more articles in American Journal of Cardiology