Contour Augmentation after Guided Bone
Regeneration with Porous Titanium Granules:
A Clinical, Histologic and Histomorphometric
Evaluation by Hadi Gholami in Modern Research in Dentistry_Open Access Dentistry and oral journals Impact factor
Abstract
Objectives: To determine clinical, histological, and
histomorphometrial results following ridge preservation via contour
augmentation with high resistance biomaterials.
Material and Methods: A 54-year-old female was
referred by her general dentist due to her crowded and periodontally
hopeless maxillary right canine and premolar teeth. Two dental implants
were surgically placed in palatal positions. The exposed implant
surfaces were covered with a slowly-resorbable, synthetic,
nanocrystalline hydroxyapatite bone substitute and overbuilding was done
with porous titanium granules (PTGs) mixed with the patient’s blood. A
long-lasting collagen membrane was fixed over the graft sites. Patient
was recalled six months later for the second-phase surgery. At that
time, a core biopsy from the augmentation site was taken.
Result: The patient’s cone beam computed tomography
scan showed that hard tissue width and height increased from 8.4 and
10.6mm to 9.2 and 12.3mm, respectively. The histological sections
revealed that new bone was bridging between the PTGs and neighboring
particles. The new bone matrix consisted of 12.41% mineralized bone
matrix and 2.82% osteoid. Concerning the osteoconductivity of PTGs,
27.0% new mineralized bone, 10.2% osteoid, and 62.8% soft tissue were
found covering the titanium particles.
Conclusion: Overbuilding the ridge via contour
augmentation with non-resorbable titanium granules could serve as a
valid approach based on optimal clinical and biological results.
Clinical Relevance: Immediate implant placement and
use of PTGs and collagen membrane all at the same time can improve
clinical periodontal parameters and implant stability in the short-term.
Keywords: Guided Bone Regeneration; Contour Augmentation; Ridge preservation; Titanium Granules
Introduction
Tooth extraction causes inevitable changes in the supporting
structures, which may lead to complications for dental implant placement
[1]. Since the 1960s, many studies have been conducted to assess the
loss of hard and soft tissues after tooth extraction, and innumerable
efforts have been made to prevent or minimize bone loss [2]. Different
methods have been suggested to preserve and reconstruct bone volume and
prevent alveolar ridge resorption following traumatic events such as
tooth extraction. The guided bone regeneration (GBR) technique has
presented promising results in repairing bone defects. Autogenous bone
grafts are considered the gold standard for GBR, due to their
osteoconductive, osteoinductive, and osteogenic properties [3,4].
Despite their high efficacy for bone reconstruction, the need for a
second surgical site, unpredictable remodeling rate and bone loss have
been regarded as their main disadvantages.
Thus, more recent studies have aimed to find a suitable alternative
for autogenous bone grafts. Bone substitutes have found their niche
within the field of dentistry and have shown promising results [5-7].
Hydroxyapatite-based materials are commonly used for this purpose.
Rothamel et al. [8] evaluated the efficacy of nanocrystalline
hydroxyapatite paste; however, he claimed that it is not efficient for
ridge preservation due to its unpredictable resorption pattern. Since
nonresorbable materials can withstand external loads and are resistant
to deformation, they can be used with high success rate in bone defect
reconstruction, especially for contour augmentation [5, 7,9-11]. The
biocompatibility of titanium has been proven in the recent years, and
its use in implant dentistry and orthopedics is widely growing. Titanium
is highly resistant to corrosion in body fluids. Furthermore, it is
considered a potentially appropriate bone substitute material due to its
nonresorbable properties [10,11].
Titanium particles can stimulate the activation of complement system
and platelets and can consequently increase the level of
platelet-derived growth factor (PDGF). PDGF has been shown to promote
bone growth, and this capability along with large surface area is
advantageous for bone reconstruction [12]. Porous titanium granules
(PTG) (Natix™, Tigran Technologies AB, Malmo, Sweden) possess these
properties and contain 700-1000μm diameter granules. The porous nature
of these granules enables bone infiltration between the particles. PTGs
consist of irregular, highly porous, nonresorbable granules of
commercially pure titanium; they were first introduced for treatment of
peri-implant defects. When implanted, the granules are able to interlock
with each other, providing a suitable environment for intra- and
inter-granule osteogenesis. A stable porous structure is formed as such,
which provides an optimal environment for bone ingrowth. Moreover, PTGs
have been successfully used in different circumstances within the
specialty of implant dentistry. They have also been suggested for
management of periodontal furcation involvement of teeth [13-15]. On the
basis of the mean total amount of regenerated bone, Tavakoli et al.
[16] reported that the use of Natix™ bone substitute with a membrane can
promote bone regeneration in the process of healing of extraction
sockets in dogs [16]. Considering the valuable characteristics
properties of titanium granules as nonresorbable materials, they may be
suitable for contour augmentation with different GBR procedures.
Therefore, the purpose of this case report was to determine
post-extraction dimensional changes following ridge preservation via
contour augmentation with PTGs and a collagen membrane for horizontal
GBR. As a second objective, the histological composition of the grafted
extraction socket was evaluated after 8 months of follow-up.
Material and Methods
Patient
A 54-year-old female was referred to our clinic complaining of
crowded and periodontally hopeless maxillary right canine and premolar
teeth. Due to excessive loss of tooth structure and severe bone loss
detected circumferentially, the suggested treatment plan included
extraction of teeth (right canine and premolars) and immediate
implantation with GBR. Initial cone beam computed tomography data showed
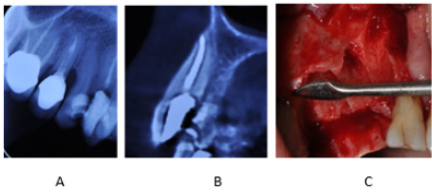
that there was no buccal bone plate over the existing teeth (Figure 1).
Figure 1: (A) Coronal plane of CBCT scan: Bone
defect present around maxillary right canine and
first and second premolars.
(B) Sagittal plane of CBCT scan: Absence of buccal
bone plate over the maxillary right canine.
(C) Clinical view of bone defect around maxillary
right canine and first and second premolars after
surgical flap elevation and tooth extraction.
Presurgical treatment
The patient’s medical and dental history showed that she had no
contraindication for dental implant surgery. The cone beam computed
tomography scans, periapical radiographs and clinical photographs were
obtained, dental cast was poured, and a clinical periodontal examination
was carried out, which included assessment of bleeding on probing
(BOP), pocket depth, and plaque index. The patient underwent scaling and
root planning and oral hygiene and plaque control instructions were
reinforced.
Surgical treatment (stage 1)
Local anesthesia was administered at the surgical site (buccally and
palatally) using 2% lidocaine with 1:100,000 epinephrine. A sulcular
incision was made extending from tooth #2 to tooth #6 with a releasing
incision at the mesial of tooth #6. A full thickness mucoperiosteal flap
was elevated to expose the labial and palatal bone plate. Interdental
papillae were preserved and reflected buccally. The hopeless teeth
(canine and premolars) were extracted via a conservative approach using
piezo surgery and periotomes. The tooth extraction sockets were then
curetted to remove all soft tissue remnants. The bone defects and
surgical sites were thoroughly irrigated with sterile saline solution.
Two dental implants (4.2x11.5mm SIC max for anterior socket and
4.0x11.5mm SIC ace for posterior socket; SIC, Switzerland) were
installed in palatal positions. Due to inadequate buccal bone, 4-8
threads of inserted implants remained exposed. The exposed implant
surfaces were covered with a slowly-resorbable, synthetic,
nanocrystalline hydroxyapatite bone substitute (NanoBoneÒ, Artoss,
Germany) and overbuilding was done beyond the resorbed or lost buccal
plate with PTGs (Natix™; Tigran Technologies AB, Malmo, Sweden) mixed
with the patient’s blood (obtained from a vein). A long-lasting collagen
membrane (SIC membrane; SIC, Germany) was fixed with two tacks (FRIOS
bone fixation system, FRIOS, Germany) at the most apical parts in the
buccal side and subsequently at the palatal side to firmly cover the
entire grafted area. The membrane covered not only the defect site, but
also a 3mm margin of sound bone at the mesial, distal, apical and
palatal.
The flaps were closed with tension-free sutures (3-0 silk) (Figure
2). Postoperatively, the patient was instructed to take 500mg
amoxicillin three times a day for a total of 10 days. Also, 0.2%
chlorhexidine gluconate mouth rinse (DarouPakhsh Holding Co., Iran) was
prescribed for plaque control, twice daily. The patient’s initial
postoperative visit was 10 days after surgery; the silk sutures were
removed. The patient received professional cleaning on a daily basis
alternately between a periodontist and general dentist for the first two
weeks. Mechanical oral hygiene practice, consisting of tooth brushing
and inter-dental brushing, was initiated by the patient on the 28th post-operative day. Post-operative care included oral hygiene reinforcement and professional plaque removal done on demand.
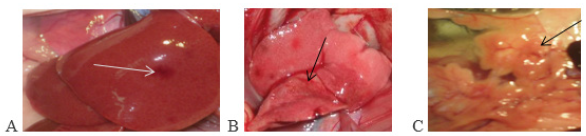
Figure 2: (A) Placement of two implants at the sites of maxillary right canine and second premolar teeth.
(B) Covering the buccal bone defect with PTGs.
(C) Placement of collagen membrane over PTGs.
(D) Returning the flap and suturing.

Surgical treatment (stage 2)
The patient was recalled six months later for the second-phase
surgery and uncovering of implants. A 2.7mm x 6.0mm trephine bur was
used to take a core biopsy from the augmentation site under copious
saline irrigation. The biopsy core was immersed in 10% formalin
solution. The patient was followed-up weekly until completion of soft
tissue healing and then monthly until healing abutment placement (Figure
3). The trephine biopsy core was sent to the Robert K. Schenk
Laboratory of Oral Histology (School of Dental Medicine, University of
Bern, Switzerland) for histologic and histometric evaluation.
Figure 3: (A) Flap elevation in the second-stage surgery.
(B) Uncovering the implants.
(C) Obtaining a core biopsy.
(D) Returning the flap and suturing.
(E) Soft tissue healing six months after surgery.
(F) Opening of healing abutment to place the temporary abutment.

Sample preparation and descriptive histology
The specimen was dehydrated in ethanol series and embedded in methyl
methacrylate. Undecalcified sections measuring ~500 microns in thickness
were cut using a low speed diamond saw under coolant (Varicut® VC-50,
Leco, Munich, Germany). After mounting the sections on acrylic glass
slabs, they were ground and polished to a final thickness of about 100µm
(Knuth-Rotor-3, Struers, Rodovre/Copenhagen, Denmark). Most sections
were stained with toluidine blue and basic fuchsine and the two most
central ground sections were used for qualitative and quantitative
analyses. A few sections were used for polarized light microscopy.
Digital photography was performed using a ProgRes® C5 digital camera
(Jenoptik Laser, Optik Systeme GmbH, Jena, Germany) connected to a Zeiss
Axioplan microscope (Carl Zeiss, Göttingen, Germany). Image processing
and morphometric analyses were performed directly under the microscope.
One experienced examiner (D.B.) performed descriptive histological
analysis.
Histomorphometry
The area fractions (%) of the newly formed mineralized bone, osteoid,
filler material 1 (PTG), filler material 2 (NanoBone) and soft tissue
(ST) occupying the biopsy volume were determined by point counting
directly under the microscope, using a square grid (distance between 6x6
test lines: 0.067mm) at x250 magnification. The percentage of PTG
surface covered with the newly formed bone (osteoconduction) was
evaluated by counting the intersections using the same grid and
magnification as mentioned above.
Result
Clinical results
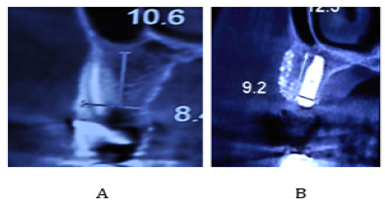
Figure 4: (A) Assessment of bone width and height
on preoperative CBCT scan of patient;
(B) Assessment of bone width and height on
postoperative CBCT scan of patient.
Our patient was a 55-year-old female with no systemic disease, who
was referred to our clinic due to failed endodontic treatment of the
maxillary right canine and premolar teeth. After extraction of the
afore-mentioned teeth due to their hopeless prognosis, two implants were
surgically placed at the sites of the maxillary right canine and second
premolar. After implant surgery, hard and soft tissue assessment was
meticulously done until prosthetic loading of implants six months after
the second-stage surgery every two months. During the follow-up
sessions, parallel periapical radiographs were obtained, scaling and
root planning was performed, and the oral hygiene instructions were
given and reinforced. Periodontal parameters including pocket depth, BOP
and pus formation were also assessed. Clinical assessment showed no
signs of inflammation around the dental implants. Probing depth in the
buccal, palatal and interproximal areas of implants was 6 mm and BOP was
present. This value was 3mm without BOP or pus after treatment. The
patient’s cone beam computed tomography scan showed that width and
height of bone increased from 8.4mm and 10.6mm before treatment to 9.2mm
and 12.3mm after treatment, respectively (Figure 4). No signs of graft
loss or gingival recession were noted around dental implants.
Histology
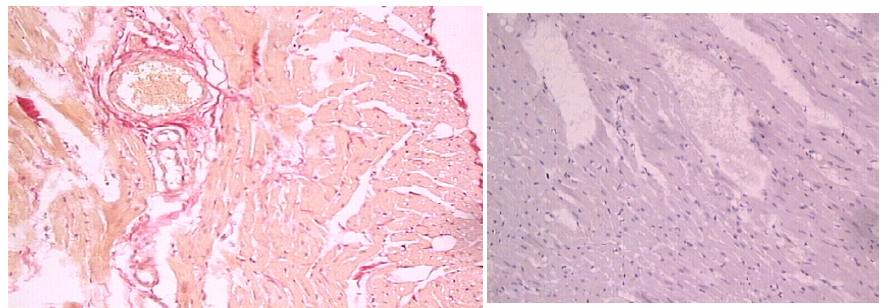
The histological sections revealed the presence of PTGs, new bone,
soft tissue and NanoBoneÒ (Figure 5-7). The PTGs were variable in shape,
size and density. Most of the new bone was lamellar bone (Figure 6a);
however, osteoid lined by osteoblasts was occasionally observed (Figure
6b). The intervening soft tissue was non-fibrous and rich in small and
medium-sized blood vessels (Figure 6a,c). New bone was observed in
direct contact with PTGs (Figure 6c) and in the pores of PTGs (Figure
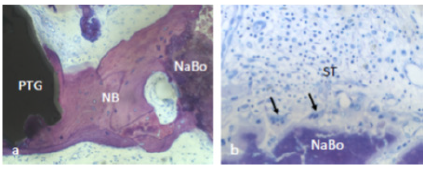
6d). Furthermore, new bone was bridging between PTGs and neighboring
NanoBoneÒ particles (Figure 7a). The NanoBoneÒ particles were lined by
new bone (Figure 7a) and few multinucleated, osteoclast-like, giant
cells (Figure 7b). Such multinucleated giant cells were not observed on
the PTG surface. The soft tissue peripheral to the NanoBoneÒ particles
was cell-rich (Figure 7b).
Figure 5: Histological section revealed the presence
of PTG: porous titanium granules, NB: new bone,
ST: soft tissue, and NaBo: remnant particles of
nano hydroxyapatite synthetic bone substitutes.

Figure 6: ST: soft tissue and NB: New bone in direct
contact with PTGs: porous titanium granules.
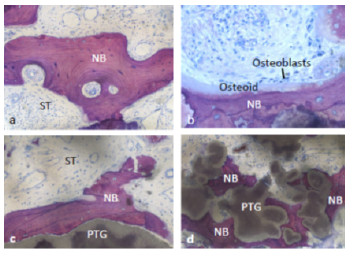
Figure 7: NB: new bone was bridging between
PTGs: porous titanium granules and neighboring
NaBo: synthetic nano hydroxyl apatite bone
substitutes (a). Multinucleated, osteoclast-like,
giant cells (arrows) and cell-rich soft tissue were
observed peripheral to Nano Bone particles (b).
Histomorphometry
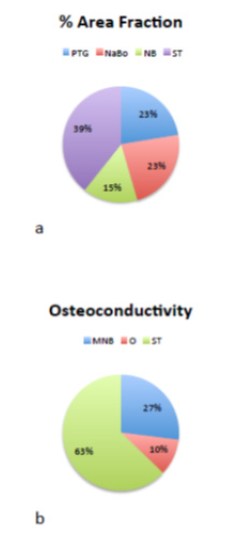
The area fractions of new bone, soft tissue, PTGs, and NanoBoneÒ were
15.2%, 39.3%, 22.4%, and 23.1%, respectively (Figure 8a). The new bone
matrix consisted of 12.41% mineralized bone matrix and 2.82% osteoid.
Concerning the osteoconductivity of PTGs, 27.0% new mineralized bone,
10.2% osteoid, and 62.8% soft tissue were found to cover the titanium
particles (Figure 8b).
Figure 8: The area fractions of NB: new bone,
ST: soft tissue, PTGs: porous titanium granules,
and NaBo: NanoBone (a). The new bone matrix
consisted of MNB: mineralized bone matrix, ST:
soft tissue, and O: osteoid (b).
Discussion
In this case study, PTGs were used to cover the bone defect in the
buccal surface of implants in the first phase of surgery. PTGs were
first introduced by Bystedt et al. [17] as an osteoconductive material
for bone grafting in sinus floor augmentation surgery [17]. Today, PTGs
are used for treatment of bone defects [18], inflammatory peri-implant
defects [19] and healing of tooth extraction sockets [20]. In our study,
some clinical periodontal parameters such as pocket depth, BOP and pus
formation were assessed at baseline and every two months after the
second-stage surgery. Wohlfahrt et al. [21] reported that clinical
attachment level and gingival recession were not significantly different
from the baseline values 12 months after treatment of grade II furcal
defects of mandibular molars with PTG, but pocket depth and gingival
index significantly decreased after treatment and BOP remained unchanged
[14]. An animal study by Wohlfahrt et al. [21] showed that probing
depth increased six weeks after treatment of grade II furcal defects
with PTGs compared to baseline, but gingival index decreased after
treatment. Horizontal probing depth was lower in group treated with PTGs
compared to the deproteinized bovine bone mineral and sham groups,
although the difference between the groups was not significant [21].
In our study, pocket depth was 6mm before treatment and BOP was
present in the areas with bone loss around teeth. After implant
placement and bone grafting, pocket depth improved to 3mm and BOP was no
longer present. For bone grafting and dental implant placement, graft
material may be applied before or simultaneously with placement of
dental implants at the site of bone loss. Applying bone graft material
prior to implant placement requires an additional surgical procedure,
and the associated risks and costs must be taken into account. In our
study, bone grafting and dental implant placement were done
simultaneously. Table 1 [22-34] lists some studies that used this
technique with different bone graft materials. In all the studies listed
in Table 1 [22-34], this technique yielded favorable radiographic and
clinical results. This technique eliminates the need for an additional
surgical procedure; however, there is a risk of implant movement and
subsequent movement of graft material. Therefore, this technique
requires high precision, and immediate loading of implants is not
encouraged and should be avoided.
Moreover, stable bone graft materials must be used. In our study,
implant loading was performed six months after the second-stage surgery
and a resorbable membrane was placed over PTGs for further
stabilization. In most of the studies listed in Table 1 [22-34], GBR
technique was used for bone grafting. The results of a review study by
Aghaloo et al. [35] showed that the implant survival rate in GBR
technique for augmentation of edentulous maxillary ridge was between
96.1-100%, which was slightly higher than that of other technique [36].
Also, collagen membrane was used in the majority of the studies cited in
Table 1 and also in our study. Collagen membrane has excellent
biocompatibility [37] and more flexibility than ePTFE and titanium mesh
[38] and does not need additional surgery for its removal. It appears
that use of membrane along with regenerative material enhances the
short-term results of bone augmentation since Delgado Ruiz et al. [39]
showed that six weeks after bone defect coverage using PTGs along with
collagen membrane, the percentage of cortical defect closure was
significantly higher than that of the PTG group without the collagen
membrane. In the PTG group without collagen membrane, evidence of tissue
inflammation was noted [40]. Roos et al. [41] stated that use of
resorbable membrane along with bone substitute had no significant
difference from the use of bone substitute alone for treatment of
peri-implantitis defects with regard to the improvement of clinical and
radiographic parameters in long-term (5 years) [42]. The above-mentioned
findings indicate that as long as the membrane has not been resorbed,
it protects bone graft materials against movement and degradation and
can therefore enhance the short-term results [43,44].
Conclusion
Tooth extraction followed by implant placement and use of
PTGs and collagen membrane all at the same time can improve
clinical periodontal parameters and implant stability in the shortterm.
Long-term randomized clinical trials with large sample
size are required in this respect. Moreover, future studies are
recommended to compare different bone graft materials for
simultaneous placement with implant insertion.
References
- Murakami Y,
Honda Y, Anada T, Shimauchi H, Suzuki O (2010) Comparative study on bone
regeneration by synthetic octacalcium phosphate with various granule
sizes. Acta Biomater 6(4): 1542-1548.
- Rickert D,
Slater JJ, Meijer HJ, Vissink A, Raghoebar GM (2012) Maxillary sinus
lift with solely autogenous bone compared to a combination of autogenous
bone and growth factors or (solely) bone substitutes. A systematic
review. Int J Oral Maxillofacial Surg 41(2): 160-167.
- Neovius E,
Engstrand T (2010) Craniofacial reconstruction with bone and
biomaterials: review over the last 11 years. J Plast Reconstr Aesthet
Surg: JPRAS 63(10): 1615-1623.
- Rubin JP,
Yaremchuk MJ (1997) Complications and toxicities of implantable
biomaterials used in facial reconstructive and aesthetic surgery: a
comprehensive review of the literature. Plast Reconstr Surg 100(5):
1336-1353.
- Gottlow J,
Nyman S, Lindhe J, Karring T, Wennstrom J (1986) New attachment
formation in the human periodontium by guided tissue regeneration. Case
reports. J Clin Periodontology 13(6): 604-616.
- Papapanou PN, Tonetti MS (2000) Diagnosis and epidemiology of periodontal osseous lesions. Periodontol 2000 22: 8-21.
- Trombelli L,
Mayfield LJ, Needleman I, Moles D, Scabbia A (2002) A systematic review
of graft materials and biological agents for periodontal intraosseous
defects. J Clinical Periodontology 29 Suppl 3: 117-135.
- Rothamel D,
Schwarz F, Herten M, Engelhardt E, Donath K, et al. (2008) Dimensional
ridge alterations following socket preservation using a nanocrystalline
hydroxyapatite paste: a histomorphometrical study in dogs. Int J Oral
Maxillofac Surg 37(8): 741-747.
- Lynch SE,
Williams RC, Polson AM, Howell TH, Reddy MS, et al. (1989) A combination
of platelet-derived and insulin-like growth factors enhances
periodontal regeneration. J Clin Periodontol 16(8): 545-548.
- Reynolds MA,
Reidy ME, Mays GL, Gunsolley JC (2003) The efficacy of bone replacement
grafts in the treatment of periodontal osseous defects. A systematic
review. Ann Periodontology 8(1): 227-265.
- Shirmohammadi
A, Chitsazi MT, Lafzi A (2009) A clinical comparison of autogenous bone
graft with and without autogenous periodontal ligament graft in the
treatment of periodontal intrabony defects. Clin Oral Investig 13(3):
279-286.
- Gholami
GA, Kadkhodazadeh M, Ardakani M, Tehranchi M, Aghaloo M, et al. (2010)
Histologic and histomorphometric evaluation of bone substitutes in
experimental defects. Res J Biol Sci 5(7): 465-469.
- Gholami G, Kadkhodazadeh M (2012) Porous titanium granules for
management of a severe intra bony defect. Journal of Clinical
Periodontology 39:134.
- Wohlfahrt JC,
Lyngstadaas SP, Heijl L, Aass AM (2012) Porous titanium granules in the
treatment of mandibular Class II furcation defects: a consecutive case
series. J Periodontol 83(1): 61-69.
- Wohlfahrt JC,
Lyngstadaas SP, Ronold HJ, Saxegaard E, Ellingsen JE, et al. (2012)
Porous titanium granules in the surgical treatment of peri-implant
osseous defects: a randomized clinical trial. International J Oral
Maxillofac Implants 27(2): 401-410.
- Tavakoli M,
Moghareabed A, Farsam T, Abbas FM, Badrian H, et al. (2012) Evaluation
of dental socket healing after using of porous titanium granules:
Histologic and histomorphometric assessment in dogs. Den Res J 9(5):
600-606.
- Bystedt H,
Rasmusson L (2009) Porous titanium granules used as osteoconductive
material for sinus floor augmentation: a clinical pilot study. Clin
Implant Dent Relat Res 11(2): 101-105.
- Wohlfahrt JC,
Monjo M, Ronold HJ, Aass AM, Ellingsen JE, et al. (2010) Porous
titanium granules promote bone healing and growth in rabbit tibia
peri-implant osseous defects. Clin Oral Implants Res 21(2): 165-173.
- Mijiritsky E,
Yatzkaier G, Mazor Z, Lorean A, Levin L (2013) The use of porous
titanium granules for treatment of peri-implantitis lesions: preliminary
clinical and radiographic results in humans. Br Dent J 214(5): E13.
- Verket A,
Lyngstadaas SP, Ronold HJ, Wohlfahrt JC (2014) Osseointegration of
dental implants in extraction sockets preserved with porous titanium
granules - an experimental study. Clin Oral Implants Res 25(2):
e100-108.
- Wohlfahrt JC,
Aass AM, Ronold HJ, Heijl L, Haugen HJ, et al. (2012) Microcomputed
tomographic and histologic analysis of animal experimental degree II
furcation defects treated with porous titanium granules or deproteinized
bovine bone. J Periodontol 83(2): 211-221.
- Demarosi F,
Varoni E, Rimondini L, Carrassi A, Leghissa GC (2016) Immediate implant
placement after removal of maxillary impacted canine teeth: A technical
note. Int J Oral Maxillofac Implants 31(1): 191-194.
- Stricker A,
Fleiner J, Stubinger S, Fleiner H, Buser D, et al. (2016) Ridge
preservation after ridge expansion with simultaneous guided bone
regeneration: a preclinical study. Clin Oral Implants Res 27(11):
e116-e124.
- Kolerman R,
Nissan J, Rahmanov A, Zenziper E, Slutzkey S, et al. (2016) Radiological
and biological assessment of immediately restored anterior maxillary
implants combined with GBR and free connective tissue graft. Clin
Implant Dent Related Res 18(6): 1142-1152.
- Jung RE,
Benic GI, Scherrer D, Hammerle CH (2015) Cone beam computed tomography
evaluation of regenerated buccal bone 5 years after simultaneous implant
placement and guided bone regeneration procedures--a randomized,
controlled clinical trial. Clin Oral Implants Res 26(1): 28-34.
- Konstantinidis
I, Kumar T, Kher U, Stanitsas PD, Hinrichs JE, et al. (2015) Clinical
results of implant placement in resorbed ridges using simultaneous
guided bone regeneration: a multicenter case series. Clin Oral Investig
19(2): 553-559.
- Covani U,
Canullo L, Toti P, Alfonsi F, Barone A et al. (2014) Tissue stability of
implants placed in fresh extraction sockets: a 5-year prospective
single-cohort study. J Periodontol 85(9): e323-e332.
- Noelken R,
Neffe BA, Kunkel M, Wagner W (2014) Maintenance of marginal bone support
and soft tissue esthetics at immediately provisionalized OsseoSpeed
implants placed into extraction sites: 2-year results. Clin Oral
Implants Res 25(2): 214-220.
- Cucchi A,
Ghensi P (2014) Vertical guided bone regeneration using
titanium-reinforced d-PTFE membrane and prehydrated corticocancellous
bone graft. Open Dent J 8: 194-200.
- Jung RE,
Fenner N, Hammerle CH, Zitzmann NU (2013) Long-term outcome of implants
placed with guided bone regeneration (GBR) using resorbable and
non-resorbable membranes after 12-14 years. Clin Oral Implants Res
24(10): 1065-1073.
- Favero G,
Botticelli D, Favero G, Garcia B, Mainetti T, et al. (2013) Alveolar
bony crest preservation at implants installed immediately after tooth
extraction: an experimental study in the dog. Clin Oral Implants Res
24(1): 7-12.
- Caneva M,
Botticelli D, Morelli F, Cesaretti G, Beolchini M, et al. (2012)
Alveolar process preservation at implants installed immediately into
extraction sockets using deproteinized bovine bone mineral-an
experimental study in dogs. Clin Oral Implants Res 23(7): 789-796.
- Shibly O,
Kutkut A, Albandar JM (2012) One-year re-entry results of guided bone
regeneration around immediately placed implants with immediate or
conventional loading: a case series. J Int Acad Periodontol 14(3):
62-68.
- Benic GI,
Jung RE, Siegenthaler DW, Hammerle CH (2009) Clinical and radiographic
comparison of implants in regenerated or native bone: 5-year results.
Clin Oral Implants Res 20(5): 507-513.
- Aghaloo TL,
Misch C, Lin GH, Iacono VJ, Wang HL, et al. (2016) Bone augmentation of
the edentulous maxilla for implant placement: A systematic review. Int J
Oral Maxillofac Implants 31 Suppl: s19-s30.
- Janner SF,
Bosshardt DD, Cochran DL, Chappuis V, Huynh Ba G, et al. (2017) The
influence of collagen membrane and autogenous bone chips on bone
augmentation in the anterior maxilla: a preclinical study. Clin Oral
Implants Res 28(11): 1368-1380.
- Wessing B,
Urban I, Montero E, Zechner W, Hof M, et al. (2017) A multicenter
randomized controlled clinical trial using a new resorbable
non-cross-linked collagen membrane for guided bone regeneration at
dehisced single implant sites: interim results of a bone augmentation
procedure. Clin Oral Implants Res 28(11): e218-e226.
- Benic GI,
Bernasconi M, Jung RE, Hammerle CH (2017) Clinical and radiographic
intra-subject comparison of implants placed with or without guided bone
regeneration: 15-year results. J Clin Periodontol 44(3): 315-325.
- Delgado Ruiz
RA, Calvo Guirado JL, Abboud M, Fernandez RMP, Mate-Sanchez JE, Negri B,
et al. (2014) Porous titanium granules in critical size defects of
rabbit tibia with or without membranes. Int J Oral Sci 6(2): 105-110.
- Benic GI, Ge
Y, Gallucci GO, Jung RE, Schneider D, et al. (2017) Guided bone
regeneration and abutment connection augment the buccal soft tissue
contour: 3-year results of a prospective comparative clinical study.
Clin Oral Implants Res 28(2): 219-225.
- Roos-Jansaker
AM, Persson GR, Lindahl C, Renvert S (2014) Surgical treatment of
peri-implantitis using a bone substitute with or without a resorbable
membrane: a 5-year follow-up. J Clin Periodontol 41(11): 1108-1114.
- Gultekin BA,
Cansiz E, Borahan MO (2017) Clinical and 3-dimensional radiographic
evaluation of autogenous iliac block bone grafting and guided bone
regeneration in patients with atrophic maxilla. J Oral Maxillofac Surg
75(4): 709-722.
- Chattopadhyay S, Raines RT (2014) Review collagen-based biomaterials for wound healing. Biopolymers 101(8): 821-833.
- Caffesse RG,
Nasjleti CE, Morrison EC, Sanchez R (1994) Guided tissue regeneration:
comparison of bioabsorbable and non-bioabsorbable membranes. Histologic
and histometric study in dogs. J Periodontol 65(6): 583-591.
For more articles in Open Access Dentistry and oral journals Impact factor