In Vitro Antimicrobial Activity of Clove Oil against Gram Negative Bacteria Isolated from Chickens by Abdulwahab Kammon in Approaches in Poultry, Dairy & Veterinary Sciences_The Journal of Poultry Science
Abstract
The use of antibiotics as growth promotors to enhance animal production is banned in many countries around the world due to antimicrobial resistance. There is a need therefore, for new alternatives to antibiotics in medicine and veterinary medicine practices.
The antibacterial activity of clove oil and some antibiotics were tested in vitro against three isolates of E. coli {1 avian pathogenic (E. coli 6.2) and 2 non-pathogenic (E. coli 6.1 and E. coli X)}, Salmonella enteritidis and Salmonella spp., by disk diffusion and minimum inhibitory concentration (MIC) methods. All bacteria tested showed intermediate susceptibility to clove oil using disk diffusion method except the non-pathogenic E.coli 6.1. The inhibition zones measured were 0mm, 12mm, 13mm, 15mm and 15mm for E. coli 6.1, E. coli 6.2, E. coli X, Salmonella enteritidis and Salmonella spp., respectively. E. coli 6.1 was resistant to Ampicillin, and Lincomycin with multi antibiotic resistance (MAR) index of 0.2. E. coli 6.2 was resistant to Ampicillin, Doxycyclin and Lincomycin with MAR index of 0.3. E. coli X was resistant to Ampicillin and Colistin with MAR index of 0.2. Salmonella enteritidis was resistant to Amoxycillin/Clavulinic acid, Ampicillin and Lincomycin with MAR index of 0.3. Salmonella spp was susceptible only to Neomycin but was resistant to 9 out of 10 antibiotics with very high MAR index of 0.9. The MICs of clove oil were 6.25mg/ml for the avian pathogenic E. coli and 3.12mg/ml for non-pathogenic E. coli and Salmonella spp. and 12.5mg/ml for Salmonella enteritidis. It is concluded that clove oil has promising antibacterial activity and more studies are needed to investigate its in vivo activity as alternative to antibiotics in poultry production. However, the mechanism of resistance of non-pathogenic E. coli 6.1 needs more investigation.
Keywords: Clove oil; E. coli; Salmonella enteritidis; Salmonella spp., Chicken; Antibiotics; Disk diffusion; MIC
Introduction
Antibiotics are extensively used as growth promoters in animal production or to control infectious diseases. Anti-microbial misuse is considered to be the most vital selecting force to antimicrobial resistance of bacteria [1]. The use of antibiotics as growth promotors to enhance animal production is banned in many countries around the world where organic production is being encouraged. There is a need therefore, for new alternatives to antibiotics in medicine and veterinary medicine practices [2].
Various plant extracts, especially essential oils, have been studied for their antimicrobial activities. The essential oils are mixture of fragrant and volatile compounds, which are usually originated from plant, and are named with the aromatic characteristics considering the origin of plant [3]. Among the important essential oils is the oil of clove (Syzygium aromaticum). Clove extract is commonly used in the food industry because of its special aroma and natural safety. In addition, the essential oil from clove also exhibited strong antibacterial properties. Antiseptic, appetite and digestion stimulant, strong antimicrobial and antifungal [4] analgesic and anti-inflammatory, anesthetic and anticarcinogenic, antiparasitic and antioxidant activities of clove and its ingredients have been reported [5].
The use of essential oils in animal production may, therefore, have a promising potential as growth promoters and treatment without the adverse effects like that of antibiotics. In vitro studies investigating the antimicrobial activity of clove oil revealed a potential antimicrobial agent for external use [6]. However, the value of clove oil to protect or cure poultry bacterial diseases has to be well investigated. Therefore, the aim of the study was to investigate the antibacterial activity of clove oil in vitro against some gram-negative bacteria isolated from poultry.
Materials and Methods
Essential oil: Clove oil (1.04g/ml, BDH Laboratory Supplies, England).
Bacteria: 3 isolates ofE. coli(1 avian pathogenic E. coli (APEC) and 2 non-pathogenic E. coli), Salmonella enteritidis, and Salmonella spp., isolated from chickens at the Department of Poultry and Fish Diseases. Faculty of Veterinary Medicine, University of Tripoli.
Antimicrobial Sensitivity Test (disk diffusion method)
The antimicrobial activity of the clove oil was evaluated against the microorganisms E.coli 6.1, E.coli 6.2 (APEC), E.coli X, Salmonella enteritidis and Salmonella spp having concentration of 1×108cfu/ml using disc diffusion method [7]. Briefly, 20ml of Mueller Hinton agar was poured in sterile petri plates and allowed to solidify. Thereafter, 0.2ml of overnight broth cultures of each microorganism was streaked on Mueller-Hinton agar (MHA) to make a complete lawn. Sterile 6 mm filter paper discs (Whatman No. 3) were soaked in the clove oil (1.04g/ml) for 30min. After completely drying of the discs at 55 ºC, they were plated on MHA. Based on growth inhibition zone diameters obtained, bacterial strains were divided into three categories i.e. resistant (>7mm), intermediate (>12mm), and susceptible (>18mm) [8]. The test was repeated twice and diameters of the inhibition zones were measured by Vernier caliber.
Susceptibility to antibiotics was also tested using disk diffusion method for Ciprofloxacin (CIP), Trimethoprim (TMP), Amoxycillin/Clavulinic acid (AMC), Sulphamethazone trimethoprim (SXT), Ampicillin (AM), Gentamycin (CN), Colistin (CT), Doxycyclin (DO), Lincomycin (MY), and Neomycin (N). The experiment was conducted in duplicate to minimize errors. All plates were incubated at 37 ºC for 24h, after that, inhibition zone was measured and the results were recorded. The multiple antibiotic resistance (MAR) index was calculated by using the formula: a/b where ‘a’ represents the number of antibiotics to which a particular isolate was resistant and ‘b’ the total number of antibiotics tested [9].
Determination of minimum inhibitory concentration (MIC) of clove oil
The dilution method was used to determine minimum inhibitory concentration (MIC) of clove oil in 96 well microtitre plates as per the method of Jorgensen et al. [10]. This method was modified by using sterile 1.5ml Eppendorf tubes instead of the microtiter plates due to drying of solution in the wells following incubation. Stock solution of clove oil (100mg/ml) was prepared by dissolving 100μl of clove oil (1.041g/ml) in 900μl DMSO: Water (4:2v/v) solvent. For every organism and the controls, a row of tubes was kept and one hundred microliters (100 μl) of sterile brain heart infusion (BHI) broth were poured in each tube. 100μl of clove oil stock solution was transferred to the first tube of every row to obtain a concentration of 50mg/ml. Thereafter, serial dilution was performed till the lowest concentration (0.02mg/ml) was obtained. The solvent used to prepare the stock solution was also tested against the microorganisms to assess the antibacterial activity of the solvent (solvent control). Then 100 μl of each microorganism having concentration of 7.0×108cfu/ml for E.coli 6.2, 9.5×108cfu/ml for E.coli 6.1, 9.7×108cfu/ml for E.coli X, 6.3×108cfu/ml for Salmonella enteritidis and 6.8×108cfu/ml for Salmonella spp were poured in all the tubes and incubated at 37 ºC for 24h. The control row which contains a serial dilution of clove (clove control) was not inoculated with any bacteria. The presence of growth in all tubes was checked by re-culturing them on MacConkey Agar and incubating again at 37 ºC for 24h. The absence of growth at a particular concentration was then taken as the MIC for particular organism.
Results and Discussion
Antimicrobial Sensitivity Test (disk diffusion method)
The results of antimicrobial sensitivity test for clove oil and other antibiotics are summarized in Table 1. Based on growth inhibition zone diameters obtained, all bacteria tested in current study showed intermediate susceptibility to clove oil using disk diffusion method except E.coli 6.1 which was resistant [8]. This is in contrary with Nuñez and Aquino [6] who demonstrated that Escherichia coli were more sensitive to clove oil.
Table 1:Result of antimicrobial activity of clove oil and some antibiotics against gram negative bacteria using disk diffusion method and MIC.
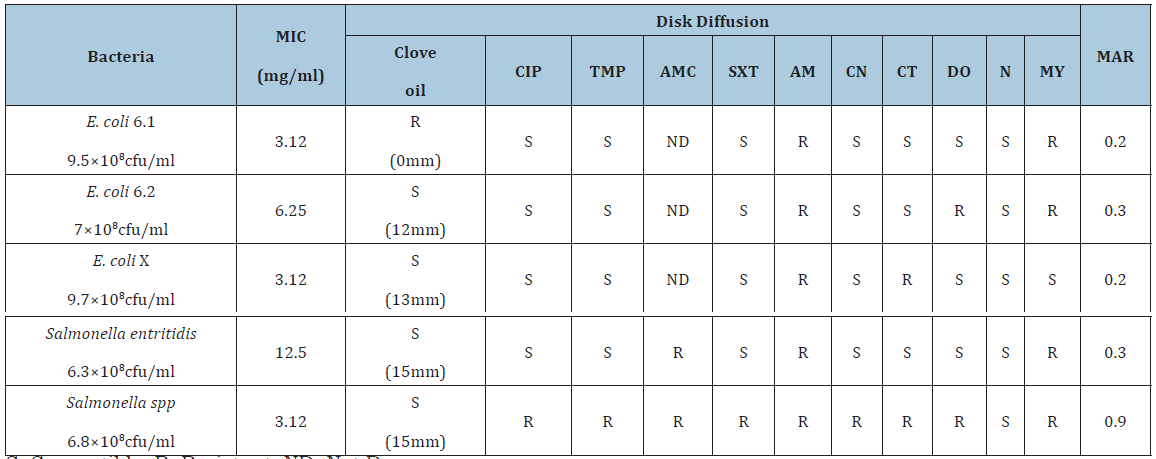
The inhibition zones measured were 0mm, 12mm, 13mm, 15mm and 15mm for E.coli 6.1, E.coli 6.2, E.coli X, Salmonella enteritidis and Salmonella spp, respectively. In another study, the antibacterial activity of clove oil against E. coli showed 19mm zone of inhibition [11]. In a study of Pathirana et al. [12], inhibition zone diameter of Gram-negative fish pathogenic bacteria ranged from 19 to 27mm and inhibition zone diameter of Gram-positive fish pathogenic bacteria ranged from 15 to 25mm in 100% (V/V) of eugenol while inhibition zone diameter of Gram-negative bacteria ranged from 16 to 20mm and inhibition zone diameter of Gram-positive bacteria ranged from 14 to 22mm at 100% (V/V) of clove essential oil. Ethanol, aqueous extracts, and essential oils of clove were analyzed for determination of antibacterial activity against 21 food borne pathogens. Inhibition zone diameters were 13-18 and 14-17 for Escherichia coli O157: H7 and Salmonella enteritidis, respectively [13].
E.coli 6.1 was susceptible to all tested antibiotics except Ampicillin and Lincomycin with MAR index of 0.2. E.coli 6.2 was resistant to Ampicillin, Lincomycin and Doxycyclin with MAR index of 0.3. E.coli X was resistant to Ampicillin and Colistin with MAR index of 0.2. Salmonella enteritidis was susceptible to Trimethoprim, Sulphamethazone trimethoprim, Gentamycin, Colistin, Doxycyclin and Neomycin but was resistant to 3 antibiotics out of 10 which were Amoxycillin/Clavulinic acid, Ampicillin, and, Lincomycin with MAR index of 0.3. Salmonella spp was only susceptible to Neomycin whereas resistant to 9 out of 10 antibiotics which were Trimethoprim, Ciprofloxacin, Amoxycillin/Clavulinic acid, Sulphamethazone trimethoprim, Ampicillin, Gentamycin, Colistin, Doxycyclin, and Lincomycin with very high MAR index of 0.9. These results are in agreement with Singh et al. [14] who addressed that the occurrence of antimicrobial resistance among zoonotic Salmonella is an increasing problem and has become a serious health hazard worldwide. The main reason of multiple antibiotic resistance level of Salmonella is widespread overuse and misuse of antibiotics in developing countries [15]. High resistance of bacteria isolated from chickens to multiple antibiotics in our study suggests that these antibiotics are widely used in the area.
Minimum Inhibitory Concentration (MIC)
The results of minimum inhibitory concentration (MIC) test for clove oil and other antibiotics are summarized in Table 1. Bacteria were isolated from all tubes containing only DMSO (solvent). This result indicates that there is no antimicrobial effect of DMSO which is in consistent with the results of Ankita [16] who studied the Effect of different solvents; dimethyl-sulfoxide (DMSO), dimethyl formamide (DMF), methanol, tween 80 and acetone on morphology, cytology and reproduction of Aspergillus flavus, A. parasiticus, A. fumigatus and A. niger. However, there was also no bacterial growth in tubes containing clove oil which were not inoculated with bacteria.
MICs recorded in mg/ml were 3.12, 6.25, 3.12, 12.5 and 3.12 for E.coli 6.1, E.coli 6.2, E.coli X, Salmonella enteritidis and Salmonella spp, respectively. The highest inhibition was for E.coli 6.1, E.coli X and Salmonella spp (3.12mg/ml) and the lowest inhibition was for Salmonella enteritidis (12.5mg/ml). The results of the current study using the clove oil correlated with the findings of other investigators. The complete essential oil of cloves has been demonstrated to be inhibitory against E. coli where the MIC was 1.25mg/ml [17]. MIC of eugenol against Salmonella typhimurium was 0.5-16mg.ml-1 [18] and 200 mg.ml-1 against E.coli [19]. Eugenol, the major constituent of clove oil was found to be such a highly effective antibacterial agent against Salmonella typhimurium that its growth was completely inhibited on media containing 100 mg/ml eugenol even when the initial inoculum was 107 cells/plate. MIC values varied depending upon the incubation period [20]. However, this inhibition was demonstrated in culture medium, not in live animals. Analyses of antibacterial properties of essential oils have been conducted by some researchers [21-23]. Essential oils show a particularly strong action against Gram-positive bacteria but it has been demonstrated that essential oils also act against Gram-negative bacteria, such as Campylobacter jejuni, Escherichia coli, Mycoplasma gallisepticum, Mycoplasma synoviae, Pseudomonas aeruginosa, Salmonella enteridis, or Klebsiella sp. [22-31]. Eugenol could potentially be used as dietary supplement to reduce cecal colonization of chickens with Salmonella enteritidis [32].
I. Presence of anti-QS activity in clove oil and other essential oils
II. has indicated new anti-infective activity.
III. Presence of anti-QS activity in clove oil and other essential oils
IV. has indicated new anti-infective activity.
The antimicrobial activity of clove is attributable to eugenol, oleic acids and lipids found in its essential oils. The mechanism of action of essential oils depends on their chemical composition, and their antimicrobial activity is not attributable to a unique mechanism but is instead a cascade of reactions involving the entire bacterial cell [33]. Presence of anti-Quorum Sensing (QS) activity in clove oil and other essential oils has indicated new anti-infective activity [34]. However, it is accepted that the antimicrobial activity depends on the lipophilic character of the components. The components permeate the cell membranes and mitochondria of the microorganisms and inhibit, among others, the membrane bound electron flow and therewith the energy metabolism. This leads to a collapse of the proton pump and draining of the ATP pool. High concentrations may also lead to lysis of the cell membranes and denaturation of cytoplasmic proteins [33,35]. In conclusion, clove oil has shown to be effective against some bacteria isolated from chickens under in vitro conditions by both disk diffusion and MIC methods. Follow-up in vivo studies are planned to evaluate these possibilities. However, further work is necessary to ascertain why E.coli 6.1 displayed resistant to clove oil when disk diffusion method was used although its MIC was 3.12mg/ml.
Acknowledgment
The authors acknowledge the Department of Chemistry, Faculty of Science, University of Tripoli for providing the DMSO.
References
9. Krumperman PH (1983) Multiple antibiotic resistance indexing of Escherichia coli to indentify high-risk sources of fecal contamination of foods. Applied Environ Microbiol 46(1): 165-170.
10. Jorgensen JH, Turnidge JD, Washington JA (1999) In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (Eds.), Antibacterial susceptibility tests: dilution and disk diffusion methods (7th edn), ASM Press, Manual of Clinical Microbiology, Washington, USA, pp. 1526-1543.
14. Singh R, Yadav AS, Tripathi V, Singh RP (2013) Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Contr 33(2): 545-548.
18. Bajpai VK, Baek KH, Kang SC (2012) Control of salmonella in foods by using essential oils: A review. Food Res Int 45: 722-773.
35. Gopi M, Karthik, Manjunathchar HV, Tamilmahan P, Kesavan M, et al. (2014) Essential oils as a feed additive in poultry nutrition. Adv Anim Vet Sci 2(1): 1-7.
https://crimsonpublishers.com/apdv/fulltext/APDV.000635.php




No comments:
Post a Comment