Endovascular Treatment of Acute Pulmonary Artery Trunk Embolism Following Thrombosis of Double Inferior Vena Cava by Tao Zhang Z in Developments in Anaesthetics & Pain Management_Journal of Research and Review
Abstract
A double Inferior Vena Cava (IVC) is a rare disease but can cause venous thrombosis and pulmonary embolism. We herein describe a patient who was admitted for evaluation of syncope. A severe pulmonary trunk embolism was found by computed tomography pulmonary angiography, but ultrasound showed no deep vein thrombosis in the lower extremity. A double IVC accompanied by floating thrombi was found during IVC angiography, and the double IVC converged above the renal veins. A temporary filter was implanted through the right internal jugular vein, and catheter-directed thrombolysis in both the pulmonary artery and IVC was performed. The patient recovered smoothly.
Conclusion: For patients with pulmonary embolism and no thrombosis in the deep veins of the lower extremities, the source of the thrombi should be clarified and the possibility of IVC malformation should be considered.
Keywords: Double inferior vena cava; Vessel anomalies; Pulmonary embolism; Catheter-directedthrombolysis
Introduction
Inferior Vena Cava (IVC) malformations are not common, but they should be carefully identified prior to IVC surgery and retroperitoneal surgery to avoid serious complications. A double IVC is a common type of IVC anomaly. A double IVC can cause anatomical and blood flow abnormalities that lead to thrombosis and secondary Pulmonary Embolism (PE). We herein report our experience with the diagnosis and treatment of a patient with acute pulmonary artery trunk embolism following thrombosis of a double IVC.
Case Report
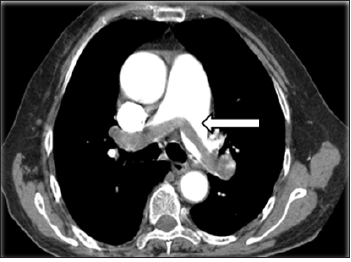
A previously healthy 46-year-old man was admitted because of sudden-onset syncope 8 hours previously. The patient had suddenly fainted 8 hours prior to presentation with no known cause and lost consciousness; however, he had no convulsions of any limbs and no fecal or urinary incontinence. The syncope lasted for about 3 minutes, and the patient gradually regained his consciousness accompanied by shortness of breath, chest distress, coughing, and expectoration. Examination upon admission revealed type I respiratory failure, and Computed Tomography (CT) pulmonary angiography showed an embolism in the main pulmonary artery; however, ultrasound examination showed no thrombus in the lower extremities (Figure 1). On physical examination, the patient’s blood pressure was 98/45mmHg, his lips were cyanosed, his heart rate was 115beats/min with a regular rhythm, and his respiratory rate was rapid (about 30breaths/min) with coarse respiratory sounds in the bilateral lungs. No swelling was seen in the lower limbs. After admission, blood gas analysis (mask-delivered oxygen at 5L/min) showed the following: pH, 7.30; PO2, 45.40mmHg; PCO2, 36.50mmHg; and SO2, 85.8%. His D-dimer concentration was 3.75mg/L. After treatment with oxygen inhalation, the patient was able to assume a supine position. His PE severity index was 126 points (grade V is defined as >125 points, which indicates very high 30-day mortality risk of (10.0-24.5) %.
Figure 1:Preoperative computed tomography showed thromboembolism in the bilateral main pulmonary arteries (arrow).
Preoperative Analysis
Peripheral thrombolysis or Catheter-Directed Thrombolysis (CDT) after IVC filter placement
The patient had acute pulmonary arterial embolism, and the thrombi were relatively large. They were considered to have been caused by the detachment of large thrombi, but no thrombus was found on an ultrasound examination of the bilateral lower extremities. Because the source of the thrombi was not clear, we were concerned that peripheral thrombolysis may result in a new thrombus that could readily detach and aggravate the PE. Therefore, the patient was scheduled to undergo IVC angiography followed by pulmonary angiography and pulmonary artery CDT.
Source of the thrombi
CT pulmonary angiography showed PE, but no swelling in the lower limbs. The ultrasound examination showed no thrombosis in the deep veins of the lower limbs. The source of the thrombi could not be identified. IVC angiography was therefore performed to determine the source of the thrombi. Because thrombi are usually seen in the IVC system, IVC angiography was the first-choice procedure. If thrombi were present, a filter could be implanted through the superior vena cava.
Operative procedure
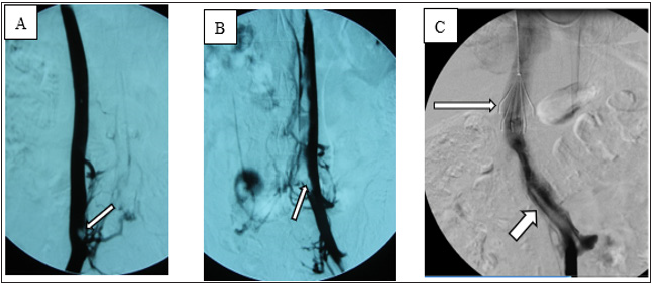
Local anesthesia was administered, and the angiography procedure was performed via puncture of the right femoral vein. Angiography showed that the IVC was relatively small in diameter and contained a filling defect. After puncture via the left femoral vein, angiography showed a double IVC (Figure 2A) and thrombosis (floating thrombi) within the IVC. The left and right IVCs merged into one branch above the renal vein. Puncture was performed via the right jugular vein, and a temporary vena cava filter was implanted (Tempofilter II; B. Braun, Melsungen, Germany) (Figure 2B & 2C). The filter was located above the junction of the two IVCs. Pulmonary artery angiography (Figure 3A) and pulmonary artery CDT were performed through the right subclavian vein. IVC CDT was performed through the left femoral vein. The thrombolysis catheter used in this patient was a Uni Fuse (AngioDynamics, Latham, NY, USA).
Figure 2: Angiography showed a double IVC with thrombosis (arrows) in both IVCs.
(A) Angiography via right femoral vein.
(B) Angiography via left femoral vein.
(C) Junction of left and right IVCs, IVC filter (long arrow) and floating thrombi in IVC (short arrow).
Postoperative treatment
After pulmonary arterial CDT and IVC filter placement, the patient was continuously infused with 500,000 IU of urokinase for 10 hours and administered low-molecular-weight dextran daily. Additionally, low-molecular-weight heparin (nadroparin at 100IU/ kg) was injected subcutaneously for anticoagulation treatment. The patient’s respiratory condition gradually improved, and his shortness of breath and chest distress disappeared. After 3 days, his blood pressure was 120/75mmHg, heart rate was 90beats/ min, and blood oxygen saturation returned to 95% to 100%. His oxygen partial pressure was normal on repeated blood gas analysis. Additionally, repeat angiography showed that the pulmonary artery thrombi had disappeared, and his blood flow was smooth (Figure 3B). After 1 month, the IVC filter was removed.
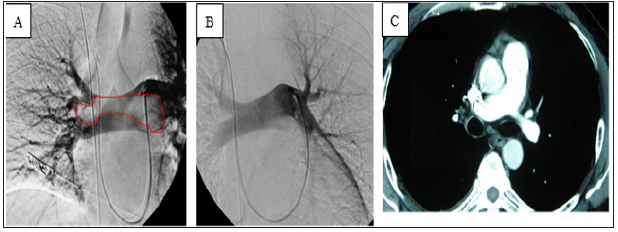
Figure 3: (A) Pulmonary angiography showed a filling defect of the main pulmonary artery (circle).
(B)After thrombolysis, the thrombi in the pulmonary arteries disappeared.
(C)Reexamination by computed tomography pulmonary angiography of the pulmonary arteries at 3 months
postoperatively showed no abnormalities.
Follow-up visits
After discharge, the patient received oral warfarin as long-term anticoagulant therapy. CT pulmonary angiography was performed again 3 months after the operation (Figure 3C). The patient was followed up for 2 years, during which time he developed no symptoms such as chest distress or shortness of breath and exhibited no lower limb venous insufficiency.
Discussion
Choice of treatment method
Generally, PE with syncope as the main manifestation is relatively severe. This patient had a massive thromboembolism in the main pulmonary arteries of the bilateral lungs, his medical condition was critical, and the source of the thrombi was unclear. Peripheral thrombolysis may result in a new thrombus in such cases, and peripheral thrombolysis therapy alone may not completely dissolve the thrombus. In our patient, angiography of the IVC was performed first, and a double IVC containing floating thrombi was found. Implantation of an IVC filter was required before thrombolysis therapy to avoid recurrence of the PE. The patient had a double IVC characterized by the joining of two IVCs between the hepatic veins and the renal veins, and thrombi had formed in the bilateral IVCs. The filter was implanted into the trunk of the IVC, and this needed to be performed via the internal jugular vein. Because the patient was young, a temporary filter was required. The Tempofilter II met these requirements.
Pulmonary arterial CDT
The treatment of acute PE includes anticoagulant therapy, thrombolytic therapy, catheter interventional surgery, surgical thrombectomy, implantation of IVC filters, and possibly other measures. The main purpose of percutaneous catheter interventional therapy is to quickly reduce the pulmonary artery pressure, restore right ventricular function, and increase systemic perfusion. In 2014, the European Society of Cardiology (ESC) guideline recommended that CDT [1] may be the first-choice treatment for patients without absolute contraindications for thrombolysis [1]. In 2019, the ESC guideline stated that surgical thrombectomy or CDT could be considered as an alternative to thrombolysis rescue, and the recommendation level was increased from IIb to IIa [2]. In the present case, the patient underwent pulmonary artery angiography via the right subclavian vein and received thrombolysis therapy by means of thrombolysis catheter implantation. After the treatment, his respiration was significantly improved, and his oxygen partial pressure returned to normal as shown by blood gas examination. After 3 days, repeat angiography showed that the pulmonary artery thrombi had disappeared and that the blood flow in the pulmonary arteries was unobstructed. The effect of thrombolysis was satisfactory in this case for two reasons: first, the CDT allowed the drugs to act quickly and intensively, and second, the CDT was conducted within 24 hours of symptom onset.
IVC malformations
The IVC can be divided into four segments: the hepatic segment,
suprarenal segment, renal segment, and infrarenal segment. IVC
malformations can be divided into three types according to the
lesion characteristics:
1. Infrarenal: left-sided IVC, duplication, pre-aortic IVC, and
absence of the infrarenal IVC
2. Renal: left retroaortic renal vein and left circumaortic
renal collar, accessory left renal vein
3. Suprarenal: absent hepatic IVC with azygos continuation,
congenital caval membranes, congenital IVC stenosis or atresia
[3,4].
IVC malformations are relatively rare, with an incidence of about 0.3% to 0.6% in the general population [5,6]. Although a large collateral circulation is present, IVC abnormalities can cause obstructions of venous reflux, leading to venous hypertension and blood flow stagnation, eventually resulting in venous thrombosis [3,7]. Some patients visit the hospital because of chronic venous insufficiency and are further diagnosed with an IVC malformation [8,9]. It is generally accepted that congenital IVC malformations should be considered in young patients with spontaneous bilateral deep venous thrombosis, and most such patients have subclinical symptoms [3,6,10]. Contrast-enhanced CT is very effective in the examination of IVC abnormalities. It can reveal the venous extension and the presence of thrombi [6,11]. Duplication is a common IVC anomaly. Notably, embryonic development of the IVC is complicated. There are three pairs of embryonic veins (postcardinal, subcardinal, and supracardinal) that form a complex anastomosis among one another, gradually regressing thereafter to form the final IVC. Failure of regression of both supracardinal veins and failure of formation of adequate connections between the primitive veins are considered reasonable explanations of double IVC formation [6,12,13]. In most cases, the abnormal left IVC is located at the level of the left renal vein, spans the aorta, and connects to the right IVC [14], and it sometimes directly connects to the left renal vein [15]. Double IVCs can be divided into asymmetric and symmetric duplication. In most cases, the right IVC is still the dominant reflux channel [6]. The clinical manifestations of a double IVC include lumbago, thrombosis, and other conditions; some patients are asymptomatic [16]. In patients with a double IVC, clinicians must be vigilant before retroperitoneal surgery; avoid massive hemorrhage, thrombosis, and other complications; determine whether a double IVC is present before IVC filter implantation; and avoid filter-related complications [6,13,17]. In the present case, two IVCs had joined above the renal vein with floating thrombi, and severe PE was present; however, the deep veins of the lower limbs were free of thrombi, and the patient had no symptoms of venous insufficiency, considering that the thrombosis was related to the venous malformation.
Conclusion
Although a double IVC with floating thrombi is rare, clinicians must still be vigilant of the possibility, and it is helpful to formulate a treatment plan. For patients with PE and no swelling in the lower limbs or thrombosis in the deep veins of the lower limbs as shown by ultrasound examination, the source of the thrombi should be clarified. Both the IVC and iliac vein should be considered as potential sources.
References
- Konstantinides SV (2014) ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35: 3145-3146.
- Konstantinides SV, Meyer G, Becattini C (2019) ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41: 543-603.
- McAree BJ, O Donnell ME, Fitzmaurice GJ (2013) Inferior vena cava thrombosis: A review of current practice. Vasc Med 18(1): 32-43.
- Alkhouli M, Morad M, Narins CR, Raza F, Bashir R (2016) Inferior vena cava thrombosis. JACC Cardiovasc Interv 9(7): 629-643.
- Ordóñez SF, Carrasco GJC, Recio BFJ, Aguilar RC, López TF, et al. (1998) Absence of the inferior vena cava causing repeated deep venous thrombosis in an adult-A case report. Angiology 49(11): 951-956.
- Spentzouris G, Zandian A, Cesmebasi A, Kinsella CR, Muhleman M, et al. (2014) The clinical anatomy of the inferior vena cava: A review of common congenital anomalies and considerations for clinicians. Clin Anat 27(8): 1234-1243.
- Fuster GMJ, Forner MJ, Lorente B, Soler J, Campos S (2006) Inferior vena cava malformations and deep venous thrombosis. Rev Esp Cardiol 59(2): 171-175.
- Sitwala PS, Ladia VM, Brahmbhatt PB, Jain V, Bajaj K (2014) Inferior vena cava anomaly: A risk for deep vein thrombosis. N Am J Med Sci 6(11): 601-603.
- Baeshko AA, Zhuk GV, Orlovskii Iu N, Ulezko EA, Savitskaia TV, et al. (2007) Congenital anomalies of the inferior vena cava: Diagnosis and medical treatment. Angiol Sosud Khir 13(1): 91-95.
- Chee YL, Culligan DJ, Watson HG (2001) Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 114(4): 878-880.
- Gayer G, Luboshitz J, Hertz M, Zissin R, Thaleret M, et al. (2003) Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol 180(3): 729-732.
- Shaw MB, Cutress M, Papavassiliou V, White S, Thompson M, et al. (2003) Duplicated inferior vena cava and crossed renal ectopia with abdominal aortic aneurysm: Preoperative anatomic studies facilitate surgery. Clin Anat 16(4): 355-357.
- Natsis K, Apostolidis S, Noussios G, Papathanasiou E, Kyriazidou A, et al. (2010) Duplication of the inferior vena cava: Anatomy, embryology and classification proposal. Anat Sci Int 85(1): 56-60.
- Sartori MT, Zampieri P, Andres AL, Prandoni P, Motta R, et al. (2006) Double vena cava filter insertion in congenital duplicated inferior vena cava: A case report and literature review. Haematologica 91(Suppl): ECR30.
- Kapetanakis S, Papadopoulos C, Galani P, Dimitrakopoulou G, Fiska A (2010) Anomalies of the inferior vena cava: A report of two cases and a short review of the literature. Folia Morphol (Warsz) 69(3): 123-127.
- Di Nicolò P, Zanoli L, Figuera M, Granata A (2016) An unusual cause of lumbar pain after physical exercise: Caval vein duplicity and its detection by ultrasound. J Ultrasound 19(4): 289-293.
- Katyal A, Javed MA (2016) Duplicate inferior vena cava filters: More is not always better. Am J Emerg Med 34(1): 115.e1-4.




No comments:
Post a Comment