Beneficial Effect of Qurs-E-Damavi, A Traditional Unani Formulation in Cyclophosphamide Induced Haematological Perturbations in Rats by Gulam Mohammed Husain in journal of traditional and complementary medicine
Abstract
Qurs-e-Damavi (DM) is a polyherbal Unani formulation. It contains Rewand Chini (Rheum emodi), Zanjabeel (Zingiber officinale), Samagh-e-Arabi (Acacia arabica) and Hira Kasees (sulftes of Iron). It is used in conditions like anaemia. The aim of the current study was to validate the use of DM and its hydroethanolic extract (DME) in cyclophosphamide induced haemotoxicity in rats for the assessment of erythropoietic activity. DM was prepared as per classical methodology. Extract (DME) was obtained from crude formulation (DM) using extraction with ethanol and water (1:1; v/v). Haemotoxicity was induced by intraperitoneal administration of cyclophosphamide 3mg/kg bw in rats for seven consecutive days. Drug treatment was started from day-8 and continued till day-22. Blood samples were analysed on day-7 and day-22 using haematology analyser. Treatment with DM at 25 and 50mg/kg bw significantly reversed haemotoxicity induced by cyclophosphamide and haematological parameters of treated groups were comparable to vehicle control except a significant decrease (p<0.01) in WBC count at DM 25mg/kg bw group. DME 10mg/kg treatment normalized Hb and PLT count, however, RBC, WBC and HCT values were still significantly lower (p<0.05) compared to vehicle control. DME 20mg/kg treatment restored all hematological parameters except a significant decrease (p<0.001) in WBC count was persisted on day-22. Treatment with DM at 25 and 50mg/kg bw restored the haematological parameters in rats induced by cyclophosphamide. DME effectively restored haematological parameters only at the dose of 20mg/kg bw. Observed effect may be exerted by synergistic effect of the phytoconstituents of DM ingredients such as Rheum emodi, Zingiber officinale, Acacia arabica and iron. Present findings validate the indication of this traditional Unani formulation in the management of iron deficiency conditions like anaemia.
Keywords: Unani; Erythropoiesis; Polyherbal formulation; Haemopoiesis; Cyclophosphamide; Myelosuppression
Abbreviations: DME: Hydroalcoholic Extract; CP: Cyclophosphamide; TED: Therapeutic Equivalent Dose; Hb: Haemoglobin, RBC: Red Blood Cell; WBC: White Blood Cell; HCT: Haematocrit; PLT: Platelet
Introduction
Qurs-e-Damavi (DM) is a polyherbal Unani formulation. It contains Rewand Chini (Rheum emodi), Zanjabeel (Zingiber officinale), Samagh-e-Arabi (Acacia arabica) and Hira Kasees (sulfates of Iron). Though this traditional Unani formulation is being used clinically since long time (based on traditional knowledge), scientific data is lacking to support definite use of this Unani formulation. Therefore, the present study is designed to evaluate the effect of Qurs-e-Damavi and its 50% hydroalcoholic extract (DME) on haematopoiesis.
Materials and Methods
Effect of Qurs-e-Damavi and its 50% hydroalcoholic extract (DME) was evaluated in cyclophosphamide (CP) induced haematological perturbations in rats.
Preparation of the formulation
Qurs-e-Damavi (DM) was prepared in the GMP certified Pharmacy Section of National Research Institute of Unani Medicine for Skin Disorders, Hyderabad as per the composition which includes Rewand Chini (Rheum emodi), Zanjabeel (Zingiber officinale), Samagh-e-Arabi (Acacia arabica) and Hira Kasees (sulphates of Iron).
50% hydroethanolic extract of DM was prepared by Drug Standardisation Research Unit of the Institute. Briefly, DM was soaked in 1:1 mixture of water and ethanol (v/v) for 24 hrs with intermittent shaking, followed by filtration. The supernatant was discarded, and filtrate was evaporated to obtain dry extract (DME) which was weighed to calculate the yield and stored in desiccators till further use.
Experimental animals
Sprague Dawley rats (220±30g; Male) were used for the present study. Animals were procured from EDARA research foundation, Hyderabad. Rats were group housed in polysulfone cages in the temperature-controlled room maintained at the temperature of 22°C ± 3°C and relative humidity of 30-70%, with a 12:12 h light/dark illumination cycle. Study was approved by Institutional Animals Ethics Committee vide protocol no. 1034/ GO/Re/S/07/CPCSEA. National guidelines of laboratory animal care (CPCSEA) were followed throughout the experiment [1]. Rats were maintained on standard diet (SDS diet, England) and water ad libitum, unless mentioned otherwise. Corn cob bedding was used for housing the animals. Only male rats were used to avoid the influence of the estrus cycle on drug metabolism and/or efficacy. Rats were acclimatized to the laboratory conditions for one week before using them for experiment.
Dose selection and study design
Therapeutic dose of DM for adult human is reported as 250mg per day. Accordingly, as per body surface area conversion method [2], Therapeutic Equivalent Dose (TED) for rat is about 25mg/kg bw per day. Therefore, present study was performed at two dose levels of DM i.e., 25 and 50mg/kg bw/day. The percentage yield of hydroethanolic extract was found to be 40.46% (w/w). Accordingly, equivalent doses of extract (DME) for rats are 10 and 20mg/kg bw/ day.
Cyclophosphamide induced haemotoxicity
Hematopoietic activity was evaluated cyclophosphamide induced haemotoxicity model in rats [3]. Haemotoxicity was induced by intraperitoneal administration of cyclophosphamide (3mg/kg bw) for 7 consecutive days. Rats were divided into six groups (6 male animals in each group) and treated as Table 1.
Table 1: Treatment for rats.
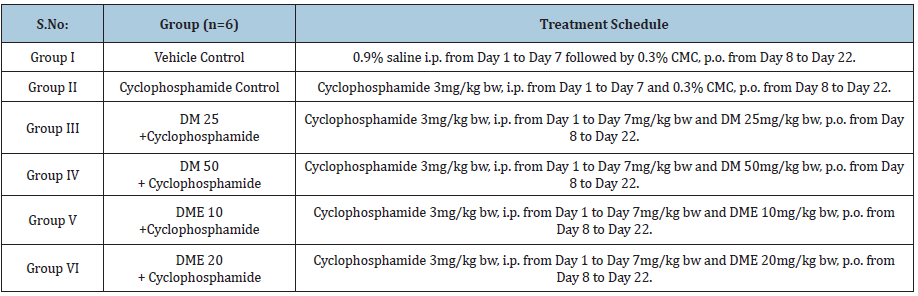
DM and DME were suspended in 0.3% CMC every day using mortar pestle. The test drugs were orally administered as an aqueous suspension at the maximum volume of 2mL/100gm bw. The control rats were administered with vehicle (i.e., 0.3% CMC) only. Test drugs or vehicle were administered via stainless steel gavage, by calculating the individual dose based on the body weight of each rat.
On day 7, blood samples were collected from the retro-orbital plexus under isoflurane anesthesia and evaluated for various blood parameters using automatic haematology analyser. Hemoglobin (Hb), red blood cell count (RBC), white blood cell count (WBC), hematocrit (HCT) and platelet (PLT) counts were analyzed. Further, blood will be collected on the 22nd day and evaluated for the same hematological parameters.
Statistical analyses
Data from the experiments was expressed as mean ± standard error of mean (SEM). The mean difference between the control and treatment groups was analysed by one-way Analysis of Variance using Graph Pad prism (version 5) Graph Pad Software, Inc., CA, USA. p value<0.05 was considered as statistically significant.
Result
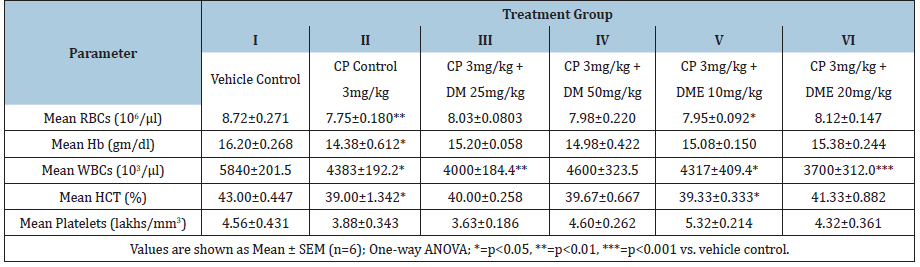
Rats treated with cyclophosphamide 3mg/kg for seven days (group II-VI) showed marked decrease (statistically significant in most of cases; refer to (Table 2) in haematological parameters such as RBC, Hb, WBC, HCT and PLT compared to vehicle control. Haematological perturbations persisted on day- 22 in cyclophosphamide control rats (group-II) and there was a significant reduction in RBC (p<0.01), Hb (p<0.05), WBC (p<0.05), and HCT (p<0.05) compared to vehicle control (Table 3). Treatment with DM at 25 and 50mg/kg bw significantly normalised these haematological parameters and all values were comparable to vehicle control except a significant decrease (p<0.01) in WBC count at DM 25mg/kg bw (4000±184.4 vs. 5840±201.5 of control). DME 10mg/kg treatment normalized Hb and PLT count, however, RBC, WBC and HCT values were still significantly lower (p<0.05) compared to vehicle control. DME 20 mg/kg treatment normalized all hematological parameters except a significant decrease (p<0.001) in WBC count was persisted on day-22 (3700±312.0 vs. 5840±201.5 of control).
Table 2: Effect of Damavi (DM) and its hydroethanolic extract (DME) on haematological parameters in cyclophosphamide- treated albino rats (after 7 days).
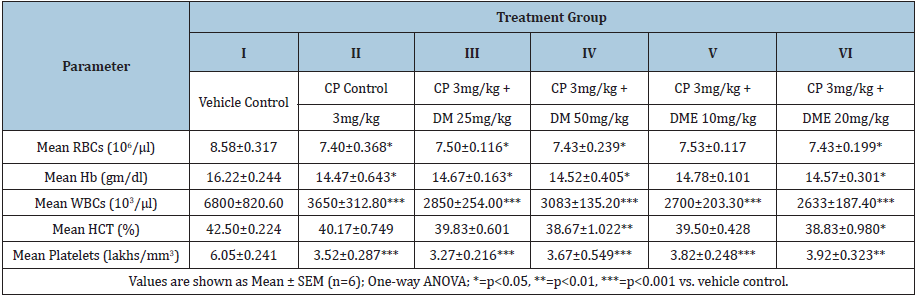
Table 3: Effect of Damavi (DM) and its hydroethanolic extract (DME) on haematological parameters in cyclophosphamide- treated albino rats (after 22 days).
Discussion
Present study was carried out to determine erythropoietic activity of traditional Unani formulation DM and its hydroethanolic extract DME. Cyclophosphamide induced model in rats was used to assess the effect of DM and DME on various haematological parameters. Cyclophosphamide induced toxicity model has been routinely used to evaluate the beneficial effect of herbal formulation in blood dyscrasia [3-5]. Cyclophosphamide is an antineoplastic drug that causes myelosuppression due to its metabolites which limits its clinical use [6]. The severity of myelosuppression increases with higher doses of cyclophosphamide. Cyclophosphamide induces myelosuppression by oxidative stress. Traditional medicines have been reported to reverse such changes [7,8].
In the present study, administration of cyclophosphamide at 3mg/kg for seven days (group II-VI) resulted in significant reduction of the RBC, Hb, HCT, WBC and PLT counts on day-7 as compared to vehicle control animals. Treatment with DM at 25 and 50mg/kg from day 8 to day 22 significantly normalised these haematological parameters. Oral administration of DME at the dose of 20mg/kg from day-8 to day-22 restored hematological alterations induced by cyclophosphamide except a significant decrease in WBC count. However, DME 10mg/kg did not effectively reversed haematological parameters induced by cyclophosphamide.
Observed beneficial effect of DM may be exerted by the presence of iron i.e., Hira Kasees (Sulphates of Iron) and atleast partly due to other constituents of DM such as ginger. It is reported that Rainbow trout (Oncorhyncus mykiss) fed with powdered ginger rhizome showed significant immune-stimulatory effect, increasing WBC, haematocrit, RBC count compared with the control group [9]. A recent study reported a significant increase in RBC, WBC, as well as the level of haematocrit and haemoglobin in fish (Cyprinus carpio; common carp) fed with ginger (Zingiber officinale) supplemented diet [10].
R. emodi another ingredient of DM has traditionally been used as diuretic, liver stimulant, purgative/cathartic, stomachic, anticholesterolemic, antitumour, antiseptic, immunomodulatory, as tonic and in menstrual disorders like dysmenorrhoea and menorrhagia (Zargar Hina). Several anthraquinone derivatives including emodin, aloe-emodin, physcion, chrysophanol, rhein, emodin glycoside and chrysophanol glycoside occur as the main chemical constituents [11,12]. R. emodi is reported as antioxidant and bioactivity-guided isolation of roots of R. emodi revealed eugenol, gallic acid, quercetin, rutin, epicatechin, desoxyrhapontigenin, rhapontigenin and mesopsin as major phenolic compounds responsible for the antioxidant activity [13]. Water soluble fraction of alcoholic extract of R. emodi is reported to have nephroprotective effect on all the proximal tubule segments possibly through antioxidant action of the tannins present in the fraction [14]. R. emodi also contains various micro and macro elements such as K, Ca, Fe, Mn, Na, Zn, Co, Li and Cu [15]. Aqueous extract of R. emodi was found to be safe up to 4000mg/kg/day in rats in a repeated dose 90-day oral toxicity study in rats [16]. Reported pharmacological profile of R. emodi including antioxidant potential clearly support the observed beneficial effect of DM in present study. A. arabica, another ingredient of DM is reported as a potent free radical scavenger and hepatoprotective and the polyphenol rich fraction is responsible for free radical scavenging activity [17,18]. Taken together, cumulative synergistic effect of individual ingredients is responsible for observed beneficial effect of this compound Unani formulation in cyclophosphamide induced haemotoxicity in rats. Findings of the study support that DM is a potentially effective therapy to overcome conditions like anaemia and leukocytopenia. Test formulation may be particularly useful as adjuvant therapy to overcome or curtail cyclophosphamideinduced damage during cancer chemotherapy. Further studies are warranted to explore the underlying mechanism DM and DME against cyclophosphamide induced toxicity.
Conclusion
Treatment with DM at 25 and 50mg/kg bw restored the haematological parameters in rats induced by cyclophosphamide. DME effectively restored haematological perturbations only at 20mg/kg bw. Observed effect may be exerted by the presence of iron and other constituents of DM such as flavonoids, terpenoids, and steroids. Present findings validate the indication of this traditional Unani formulation in the management of iron deficiency anaemia and DM could be a potential formulation for erythropoietic activity.
Acknowledgement
The authors would like to record their profound gratitude to Prof. Asim Ali Khan, Director General, Central Council for Research in Unani Medicine, Ministry of AYUSH, Government of India, for providing the necessary infrastructure and facilities. We would like to acknowledge Pathology Laboratory and Pharmacy section staff for their constant support.
References
- Anonymous (2018) Compendium of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Ministry of Environment, Forest & Climate Change, Government of India, India.
- Reagan SS, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22(3): 659-661.
- Pawar RS, Jain AP, Kashaw SK, Singhai AK (2006) Haematopoietic activity of asteracantha longifolia on cyclophosphamide-induced bone marrow suppression. Indian J Pharm Sci 68(3): 337-340.
- Udintsev SN, Schakhov VP (1991) Decrease of cyclophosphamide haematotoxicity by Rhodiola rosea root extract in mice with Ehrlich and Lewis transplantable tumors. Eur J Cancer 27(9): 1182.
- Cengiz M (2018) Hematoprotective effect of boron on cyclophosphamide toxicity in rats. Cell Mol Biol 64(5): 62-65.
- Ayhanci A, Yaman S, Appak S, Gunes S (2009) Hematoprotective effect of seleno-L-methionine on cyclophosphamide toxicity in rats. Drug Chem Toxicol 32(4): 424-428.
- Patra K, Bose S, Sarkar S, Rakshit J, Jana S, et al. (2012) Amelioration of cyclophosphamide induced myelosuppression and oxidative stress by cinnamic acid. Chem Biol Interact 195(3): 231-239.
- Meng X, Rong RH, Yu JZ, Keiichi A, Hiroshi K (2014) Effects of carnosine on cyclophosphamide-induced hematopoietic suppression in mice. Am J Chin Med 42(1): 131-142.
- Haghighi M, Rohani MS (2013) The effects of powdered ginger rhizome (Zingiber officinale) on haematological and immunological parameters of rainbow trout Oncorhynchus mykiss. Journal of Medicinal Plant and Herbal Therapy Research 1: 8-12.
- Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Doan HV (2020) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum, and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol 99: 267-273.
- Zargar BA, Masoodi MH, Ahmed B, Ganie SA (2011) Phytoconstituents and therapeutic uses of Rheum emodi ex Meissn. Food Chem 128(3): 585-589.
- Rehman H, Begum W, Anjum F, Tabasum H (2014) Rheum emodi (Rhubarb): a fascinating herb. Journal of Pharmacognosy and Phytochemistry 3(2): 89-94.
- Singh PP, Ambika, Chauhan SM (2013) Activity-guided isolation of antioxidants from the roots of Rheum emodi. Nat Prod Res 27(10): 946-949.
- Alam MM, Javed K, Jafri MA (2005) Effect of Rheum emodi (Revand Hindi) on renal functions in rats. J Ethnopharmacol 96(1-2): 121-125.
- Singh P, Negi JS, Rawat MS, Nee PGJ (2010) Quantification of mineral elements of Rheum emodi (Polygonaceae). Biol Trace Elem Res 138(1-3): 293-299.
- Ye BG, Feng Y, Wang S (2014) Scientific evaluation of the acute toxicity and 13-week subchronic toxicity of Rheum emodi rhizome extracts in Sprague Dawley rats. Food Chem Toxicol 66: 278-285.
- Sundaram R, Mitra SK (2007) Antioxidant activity of ethyl acetate soluble fraction of Acacia arabica bark in rats. Indian J Pharmacol 39(1): 33-38.
- Aadil KR, Barapatre A, Rathore N, Pottam S, Jha H (2012) Comparative study of in vitro antioxidant and antidiabetic activity of plant extracts of Acacia arabica, Murraya koeingii, Catharanthus roseus and Rouwolfia serpentina. International Journal of Phytomedicine 4(4): 543-551.




No comments:
Post a Comment