Acrylamide Induced Toxicity in Seminal Vesicles and its Amelioration by Vitamin E Supplementation: A Histological Study by Nisreen A Rajeh in Developments in Clinical & Medical Pathology_journal of medical & surgical pathology impact factor
Abstract
Acrylamide (ACR) is an odorless, water soluble vinyl monomer which exhibits several toxicological properties that affects almost all the major organ systems of human body. Reproductive toxicity due to ACR seems to be more pronounced in males than females. To elucidate the effect of ACR on reproductive system in males we designed the current study investigating the histopathological changes in seminal vesicles of male Wister rats. We also explored the ameliorative potential of vitamin E on toxicity induced by ACR in seminal vesicles. The experimental rats were divided into four groups. Group I served as control, Group II were treated with ACR, Group III with ACR+ vitamin E and Group IV was treated with only vitamin E. Our results clearly depicted noticeable changes in the histology of seminal vesicle of rats i.e., disorganization of mucosal folds with desquamation, necrosis of covering epithelium, cracked luminal secretions, loss of compactness of muscle layer and atrophied muscles. These changes were constructively reversed in Group III which was supplemented with vitamin E while ACR was also present. Distinguishable changes were observed in this group as evident by preservation of mucosal fold architecture and columnar covering epithelium of seminal vesicle. The muscle layer showed potential improvement in some rats but did not completely return to its normal compact appearance in others. Thus, our study elucidated the deleterious effect of ACR on seminal vesicles for which vitamin E acted as an ameliorative agent.
Introduction
Acrylamide (ACR) is a potent toxicant that is primarily released
during industrial processes
of polymer manufacturing due to hydration of acrylonitrile [1]. Another
major source of
ACR toxicity is its generation in some carbohydrate rich foods such as
potatoes, breads and
cookies that are cooked at high temperatures (120 ℃ or above) [2,3].
When these foods are
subjected to extreme heat, a reaction between amino acids such as
asparagine and reducing
sugars such as glucose/fructose present in these foods occurs which
causes the release of
ACR (Maillard reaction) [3,4]. Whether being a dietary or occupational
exposure, ACR is
known to inflict serious toxicological effects in human body. Various
studies in animal models
have been conducted to mark the potential toxicological effects of ACR.
ACR is profoundly
reported to cause neurotoxicity, nephrotoxicity, carcinogenicity,
genotoxicity, developmental
anomalies, and reproductive defects [5-7]. The Scientific Committee on
Toxicity, Ecotoxicity
and the Environment (SCTEE) reports ACR to exhibit genotoxic effects in
germ cells as well as
in reproductive system of males as well as females [8]. Studies
regarding reproductive toxicity
of ACR have elucidated many histopathological changes in various organs
and components of
the reproductive system, especially in males. Some of the well observed
anomalies caused by
ACR in male reproductive system include testicular epithelial tissue
degeneration, decrease
in epididymal sperm reserves, histopathological lesions in testes,
decrease in sperm count
and motility and abnormalities in sperm morphology [5,9]. Even though
numerous studies
have pointed towards a deleterious effect of ACR on sperm production and
morphology, none
of them have explored the effect of direct ACR exposure on seminal
vesicles [10,11]. Seminal
vesicles are the glands that are partly responsible for production of
the seminal fluid which provides a thriving environment to the sperms.
The underlying cause
of toxicological alterations by ACR is the generation of oxidative
stress that leads to changes of some major macromolecules such
as proteins and DNA. However, further mechanisms that alter the
functioning of reproductive system on exposure to ACR need to be
investigated. Experimental studies in rats have shown that once
ACR enters the body, it generates glycidamide, which is a more
harmful metabolite than ACR itself [12]. This reaction is mediated
by enzyme cytochrome P450 E1 (CYP2E1) [13]. Glycidamide is
known to have a high potential for causing DNA as well as protein
damage as compared to the unmetabolized ACR [14]. In the process
of generation of glycidamide by CYP450 E1 from ACR, many free
radicals are also released that consequently lead to a build-up
of oxidative stress, causing lipid peroxidation and alterations in
essential proteins required for normal metabolism and functioning
of a cell [15].
Thus, considering the capability of ACR to generate oxidative
stress, antioxidant compounds such as Vitamin E (alphatocopherol),
could be explored as a potential ameliorative agent
against ACR induced toxicity. Vitamin E or alpha-tocopherol is a
type of fat-soluble vitamin found frequently in the cell membranes.
It exhibits strong antioxidant properties which inhibit lipid
peroxidation produced by the free superoxide and hydroxyl radicals
[15]. Many studies have revealed the protective role of vitamin E in
sperm cell against the damages of Reactive Oxygen Species (ROS)
along with improvement in sperm motility as well as reproductive
function [16,17]. As ACR is known to induce reproductive toxicity
by affecting sperm morphology, motility or sperm count, we try to
explore the cause of this alteration by conducting a histopathologic
study of seminal vesicles on being exposed to ACR, since these
glands play a major role in maintenance of sperm health. Following
this we investigate vitamin E as a potential attenuating agent of ACR
induced toxicity in seminal vesicles. To the best of our knowledge,
this is the first study that directly investigates the effect of ACR on
seminal vesicles and its amelioration by vitamin E.
Materials and Methodss
Materials
The plus one acrylamide (PAGE) grade of ˃99.95 purity was procured from Pharmacia Biotech (Upsala, Swedan). Vitamin E (DL-α-tocopherol Acetate) was procured from Sigma-Aldrich (Steinheim-Germany). All other materials and chemicals used in the study of molecular biology grade were procured from BHD laboratory supplies (Analar®, England).
Animals and Treatment: Forty-nine adult male Wister rats were purchased from King Fahad Medical Research Centre (KFMRC), Jeddah, Kingdom of Saudi Arabia (KSA). All animal care procedure and treatments were carried out at KFMRC. On arrival these rats were 60 days old and weighed 250±20 g. Four rats were housed per polypropylene cage with bedding made of wood shavings. The environment around the rats was controlled throughout the experiment as relative humidity of 40-65%, temperature 22±2 °C and 12 hours/12 hours light/dark cycles. The rats were provided with adequate amount of tap water and were fed laboratory chow. The animals and study design were approved by the Unit of Biomedical Ethics, King Abdulaziz University (KAU), Medical College, Jeddah, KSA. The guidelines laid by KAU follow the national and international laws and policies (National Institutes of Health Guiding Principles on the Care and Use of Laboratory Animals, USA). The experimental rats were left to adapt to the environment for 3 days before initializing the dosing. The rats were clustered into four groups (n=7). Group I was of normal control rats which were provided with tapped water and laboratory chow during the complete experimental period. Using orogasteric needle, Group II rats were treated with only acrylamide (45mg/kg bw/day) by oral gavage which was selected as effective dose for inducing ACR toxicity in our other studies as well [10]. Group III rats were administered with both acrylamide + vitamin E (200mg/kg bw/ day). Group IV was the drug control group for vitamin E treatment where the rats were given vitamin E alone (200mg/kg bw/day). This experiment was performed for 5 consecutive days. All rats were checked and weighed daily for any abnormal behavior or sudden death in experiment and recovery period. After a recovery period of 1-day post cessation of ACR the rats were sacrificed by cervical dislocation and both seminal vesicles were isolated for further experimental assessment.
Histopathology
Tissue preparation and histopathological examination: The section of seminal vesicle of all rats were isolated and fixed immediately by 10 % natural buffered formalin for 24 hrs. Tissues were then processed using automatic tissue processor (Shandon, England) by using standard laboratory procedures for histology. Tissues were briefly embedded in paraffin blocks, sectioned, and then stained with Hematoxylin and eosin stain (H&E). Tissues were examined for any histological changes using light microscopy (Olympus BX51TF) at 10X, 20X, 40X magnification and representative images were captured with Olympus DP 72 camera.
General observation: The Group II experimental rats that were treated with only acrylamide had a rough coat and showed signs of aggression and rough coat. The intake of food and water was also apparently reduced. Improved food and water intake were noticed in Group III which received a combined treatment of acrylamide with vitamin E. A normal food and water intake were also observed in Group IV rats who received only vitamin E. Throughout the experimental period, no symptoms of illness or mortality were observed in any of the groups (control as well as experimental).
Histopathology of seminal vesicle sections of control group and drug-control groups (Vit E alone): The control group which was fed only with laboratory chow and tap water during the experiment showed normal layers of seminal vesicle tissue, intact mucosal folds covered by columnar epithelium having highly basophilic and compact smooth muscle layers. The secretion within the lumen was observed to be homogenous and highly acidophilic (Figure 1a). Similarly, in the group treated with vit E alone, intact mucosal layers with normal epithelium could be seen. The muscular layers of these seminal vesicles were also observed to be well organized.
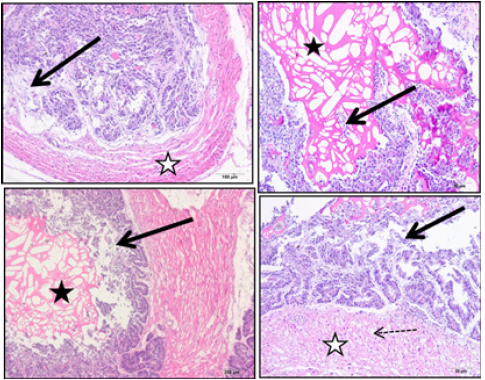
Effects of acrylamide on seminal vesicle (Group II) The Group II rats that were treated only with acrylamide showed visible changes in histopathological sections of seminal vesicle of experimental rats. Marked disorganization of mucosal folds with desquamation and necrosis of covering epithelium could be observed in different regions of seminal vesicle, the luminal secretions were noticed to be cracked. The examined muscle layer showed a loss of its compact structure, being atrophied, and appeared widely separated (Figure 2).
Figure 1:(a) The control group showing section of seminal vesicle stained with H and E stain. Black arrows indicate intact mucosal folds. Presence of highly basophilic columnar epithelium (dotted arrows). White stars indicate compact smooth muscle layers and black star indicates homogenous and highly acidophilic lumen secretion. (b) Group IV treated with vitamin E alone showing section of seminal vesicle stained with H and E stain. Low (x100x200) and high (x400) magnification photographs of rat seminal vesicle of Vitamin E group. White arrows indicate intact mucosal layers, ML indicates muscle layers and black arrows represent the lining epithelium.

Figure 2:Group II treated with acrylamide showing section of seminal vesicle stained with H and E stain. Black arrows indicate disorganized mucosal folds with necrosis and desquamation of covering epithelium. Black stars indicate cracked luminal secretions. White star indicates widely separated and atrophied muscle layer with lose compact structure.
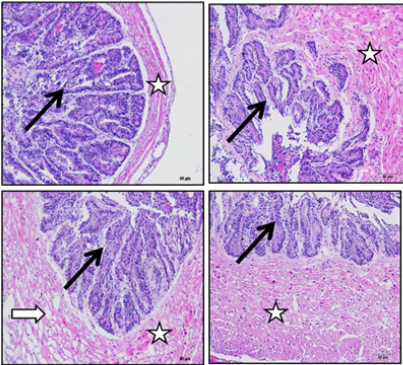
Effects of acrylamide + vitamin E on seminal vesicle (Group III): The Group III rats that were treated with acrylamide + vitamin E showed visible changes in sections of seminal vesicle of experimental rats. The results showed evident preservation of mucosal fold architecture and the covering columnar epithelium of seminal vesicle. The muscle layer showed potential improvement in some rats as compared to the rats receiving only ACR. However, on the other hand, the muscle layer of a few rats did not completely return to its normal compact appearance (Figure 3).
Figure 3: Group III treated with acrylamide + vitamin E showing section of seminal vesicle stained with H and E stain. Black arrows indicate preservation of mucosal fold architecture, columnar covering epithelium. White stars indicate potential improvement in muscle layer in some experimental rats. White arrow indicates incomplete return of muscle layer to its normal compact appearance in some experimental rats.
Discussion
Acrylamide (ACR) is an infamous toxicant known to have
negative implications on most of the organ systems of a human
body. It has been reported to act as neurotoxicant, reproductive
toxicant and carcinogen in animal models. Monomeric ACR has been
shown to cause cellular damage in both nervous and reproductive
systems and tumors in specific hormonally responsive tissues [18].
Reproductive toxicity caused by ACR has been a subject of research
dating long back. Reproductive toxicity due to ACR is found to have
many serious implications, especially in males, primarily affecting
the motility, count, and morphology of sperms [19]. However, the
exact mechanism leading to this phenomenon still needs to be
identified. Thus, this study aims at investigating the toxicity of ACR
in seminal vesicles and the role of vitamin E in its mitigation. The
present study revealed that rats treated with 45mg/kg bw/day of
ACR for 5 consecutive days became aggressive and developed rough
coat. Consequently, their food and water intake decreased. A similar
behavior has been reported in our previous studies as well [7,12].
This study clearly shows that ACR causes reproductive toxicity that
affects seminal vesicles as evident by visible changes in the vesicular
sections of experimental rats. The histological examination of the
covering epithelium showed marked disorganization of mucosal
folds with desquamation and necrosis, even the muscle layer lost
its compactness and showed signs of atrophy. Many studies have
been conducted previously to investigate the effect of ACR on male
reproductive system in rats, however, none of them have focused on
the seminal vesicles [20-23]. A study by Al-Karim et al., showed that
ACR in low dose can produce structural changes in reproductive
system of both female and male rats. They reported damage of
germ cell layers and DNA, histological changes leading to sperm
deformity as well as a decrease in seminiferous tubules of male rats
on exposure to ACR. Since the histological changes were found in
many other reproductive organs as well, this could be correlated
with our findings [24]. Similar findings were also reported where
a damage at histological and ultrastructural level was observed
because of ACR induced toxicity in testis of the rats in the form of
degeneration of the tissue and arrested spermatogenesis. Decreased
diameter of seminiferous tubules and decreased epithelial height
were also reported, however, these changes were improved by
vitamin E treatment [25].
The present study also revealed that the rats treated with
both ACR and vitamin E for 5 consecutive days showed noticeable
changes in their seminal vesicles as well. However, there was a
pronounced restoration of mucosal fold architecture, covering
columnar epithelium of seminal vesicles and muscle layer in
contrary to the ones treated with ACR only. The rats of this
experimental group (ACR + vit E) also showed improved food and
water intake. These findings correlate with the results of the study
carried out by Erdemli et al. They reported that damages indicated
by histo-morphological changes in reproductive organs of rats were less
when ACR was administered in combination with vitamin E as
compared to when ACR was administered alone thus, signifying
the healing effect of vitamin E. Their observations included
the restoration of seminiferous tubules organization, initiating
maturation of germ layer and decline of interstitial oedema [26].
Thus, histopathological examination of seminal vesicles in our
study clearly demonstrated that exposure of rats to ACR has
significant adverse effects on their reproductive system, however,
these undesirable changes could be ameliorated by administration
of vitamin E. This damage by could be attributed to the oxidative
stress generated by ACR along with an imbalance in oxidant/
antioxidant ratio, generation of glycidamide, shift towards oxidants
and lipid peroxidation [26]. Vitamin E is known to be a potent
antioxidant that protects against oxidative stress by inhibiting
propagation of ROS reactions and lipid peroxidation, which could
be one of the reasons that it showed a significant protective effect
against ACR toxicity on seminal vesicles in our study as well. Our
observations fall in line with this underlying principle as well as
with other studies reporting that ACR induced harmful effects on
male reproductive organs have been considerably improved by use
of vitamin E [27,28]. Numerous studies have reported the protective
effect of vitamin E against ACR induced toxicity in various /other
organs such as kidneys, brain, testis, skeletal muscles etc (26, 30-
32). In addition to this, research has suggested that using vitamin
E in rats exposed to toxins such as ACR may cause a reversal of
the atrophies [7,24]. To the best of our knowledge, this is the first
study that marks ACR as a potent toxin affecting seminal vesicles of
rats with vitamin E acting as an ameliorative agent of such toxicity.
In conclusion, this study states that reproductive toxicity due to
ACR is well pronounced in seminal vesicles accompanied by many
histopathological changes. These histopathological changes could
be constructively reversed by vitamin E supplementation in most
of the cases with just a few exceptions. Thus, vitamin E proved to be
an effective ameliorative agent which can protect seminal vesicles
from the deleterious effects of ACR toxicity. Further studies need to
be carried out on a larger sample size investigating the mechanisms
of ACR toxicity and its amelioration by vitamin E on a molecular
level so that it could pave a way for utilization of vitamin E as a
protective drug against ACR induced toxicity.
Acknowledgement
I highly appreciate the kind help of Professor Soaad Shaker for conducting histopathological studies and helping me in reading and examination of slides.
References
- Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419(6906): 448-449.
- Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide a review. J Agric Food Chem 51(16): 4504‐4526.
- Lineback DR, Coughlin JR, Stadler RH (2012) Acrylamide in foods: a review of the science and future considerations. Annual Review of Food Science and Technology 10(3): 15-35.
- Knol JJ, Linssen JPH, Van Boekel MAJS (2010) Unravelling the kinetics of the formation of acrylamide in the Maillard reaction of fructose and asparagine by multiresponse modelling. Food Chemistry 120(4): 1047-1057.
- Wang H, Huang P, Lie T, Li J, Hutz RJ, et al. (2010) Reproductive toxicity of acrylamide-treated male rats. Reproductive Toxicology 29(2): 225-230.
- Exon JH (2006) A review of the toxicology of acrylamide. Journal of Toxicology and Environmental Health 9(5): 397-412.
- Rajeh NA, Dhaheri NM (2017) Antioxidant effect of vitamin E and 5-aminosalicylic acid on acrylamide induced kidney injury in rats. Saudi Med J 38(2): 132‐137.
- Shipp A, Lawrence G, Gentry R, Macdonal T, Bartow H, et al. (2006) Acrylamide: review of toxicity data and dose response analysis for cancer and non-cancer effects. Crit Rev Toxicol 36: 481-608.
- Ma Y, Shi J, Zheng M, Liu J, Tian S, et al. (2011) Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health 27(7): 617‐627.
- Yue D, Yan L, Luo H, Xu X, Jin X (2010) Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim Reprod Sci 118: 217-222.
- Ghanayem BI, Witt KL, El Hadri L, Hoffler U, Kissling GE, et al. (2005) Comparison of germ cell mutagenicity in male CYP2E1-null and wild-type mice treated with acrylamide: evidence supporting a glycidamide-mediated effect. Biol Reprod 72: 157-163.
- Rajeh N, Ali H, ElAssouli S (2014) Protective effects of 5-aminosalicylic acid on acrylamide toxicity in the testis and blood leukocytes of the rat. Kuwait Med J 46(1): 32-43.
- Ghorbel I, Maktouf S, Kallel C, Ellouze Chaabouni S, et al. (2015) Disruption of erythrocyte antioxidant defense system, hematological parameters, indication of proinflammatory cytokines and DNA damage in liver of coexposed rats to aluminum and acrylamide. Chem Biol Intundereract 236: 31e40.
- Besaratinia A, Pfeifer GP (2007) A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 28: 519-528.
- Yousef MI, Demerdash FM (2006) Acrylamide induced oxidative stress and biochemical perturbations in rats. Toxicology 219(1e3): 133e141.
- Zubair M (2017) Effects of dietary vitamin E on male reproductive system. Asian Pacific Journal of Reproduction 6(4): 145.
- Hsu PC, Liu MY, Hsu CC, Chen LY, Guo YL (1998) Effects of vitamin E and/or C on reactive oxygen species related lead toxicity in the rat sperm. Toxicology 128(3): 169-179.
- Rajeh NA, Bainmahfuz FR, Alamri SM, Khan DM, Alhindi AF (2017) Protective Role of 5-Aminosalicylic acid and Vitamin-E against the acrylamide induced neurotoxicity in Rats. Am J Pharm Health Res 5(4).
- Rahangadale S, Jangir BL, Patil M (2012) Evaluation of protective effect of vitamin e on acrylamide induced testicular toxicity in wister rats. Toxicol 19(2): 158‐161.
- Yuxin Ma, Jing Shi, Meige Zheng, Jing Liu, Sumin Tian, et al. (2011) Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health 27(7): 617-627.
- Wang H, Ge JY, Zhou ZQ, Wang ZC, Shi FX (2007) Oral acrylamide affects the development and reproductive performance of male rats. Zhonghua Nan Ke Xue 13(6): 492-497.
- Manokaran K, Saleena UV, Karthik G, Kamath K, Yekula PK, et al. (2018) Effect of acrylamide on reproductive organs of adult male wister rats. J Clin Diag Res 12(11): EF01-EF05.
- Tyl RW, Marr MC, Myers CB, Ross WP, Friedman MA (2000) Relationship between acrylamide reproductive and neurotoxicity in male rats. Reprod Toxicol 14: 147-57.
- ALKarim S, ElAssouli S, Ali S, Ayuob N, ElAssouli Z (2015) Effects of low dose acrylamide on the rat reproductive organs structure, fertility and gene integrity. Asian Pacific Journal of Reproduction 4(3): 179-187.
- Hasanin NA, Sayed NM, Ghoneim FM, Sherief SA (2018) Histological and ultrastructure study of the testes of acrylamide exposed adult male albino rat and evaluation of the possible protective effect of Vitamin E intake. Journal of Microscopy and Ultrastructure 6(1): 23.
- Erdemli Z, Erdemli ME, Turkoz Y, Gul M, Yigitcan B, et al. (2019) The effects of acrylamide and Vitamin E administration during pregnancy on adult rats’ testis. Andrologia e13292.
- Makker K, Agarwal A, Sharma R (2009) Oxidative stress & male infertility. Indian J Med Res 129: 357-367.
- Momeni H, Soleimani Mehranjani M, Abnosi M, Mahmoodi M (2009) Effects of vitamin E on sperm parameters and reproductive hormones in developing rats treated with para-nonylphen ol. Iran J Reprod Med 7: 111-116.
- Ghanayem BI, Witt KL, Kissling GE, Tice RR, Recio L (2005) Absence of acrylamide induced genotoxicity in CYP2E1-null mice: evidence consistent with a glycidamide-mediated effect. Mutat Res 578: 284-297.
- Erdemli ME, Turkoz Y, Altinoz E, Elibol E, Dogan Z (2016) Investigation of the effects of acrylamide applied during pregnancy on fetal brain development in rats and protective role of the vitamin E. Hum Exp Toxicol 35(12): 1337-1344.
- Rasha H, Fatma MG (2015) The impact of vitamin E against acrylamide induced toxicity on skeletal muscles of adult male albino rat tongue: Light and electron microscopic study. Journal of Microscopy and Ultrastructure 3(3): 137-147.
- Mehmet EE, Zeynep A, Mehmet G, Birgul Y, Harika G, et al. (2018) The effects of acrylamide and vitamin E on kidneys in pregnancy: an experimental study. The Journal of Maternal-Fetal & Neonatal Medicine 32(22): 3747-3756.




No comments:
Post a Comment