Ixodidae Ticks of Bovine; Prevalence and Major Species Identification in Soddo Zuria Districts of Wolaita Zone, Ethiopia by Biruk Alemu in Approaches in Poultry, Dairy & Veterinary Sciences_The Journal of Poultry Science
Abstract
The distribution and abundance of cattle tick species in Soddo Zuria woreda, Wolaita zone, was studied over a period from November 2016 to April 2017. Adult ticks were collected from seven main body regions of 501 cattle which were under an extensive management system. Out of the total of 501 cattle examined, 327 (65.27%) were found to be infested by one or more tick species. In this study, 3290 adult ticks were collected from the animal body parts and identified to genera and species level. Four tick species of three genera (Amblyomma, Boophilus, and Rhipicephalus) were identified. The relative prevalence of each species was Boophilus decolaratus (45%), Amblyomma varigatum (23%), A. cohaerence (17%), and Rhipicephalus evertsi-evertsi (13%). The risk factor like sex of cattle did not show significant association with the infestation rate but there was an association with age, breeds and body conditions. The prevalence of tick infestation in poor body condition (75%), medium body condition (61%) and good body condition (55%) were found to be statistically significant (p<0.05) among the three groups of body conditions.
The prevalence of tick infestation was found to be statistically significant (p<0.05) among the three breeds, with the highest prevalence in local breeds (70%) than both exotic (62%) and cross breeds (54%). The result indicated that the favorable predilection sites of Amblyomma species were ventral body and perineum. B. decolaratus preferred dewlap, udder/scrotum, belly, leg/tail, head, and perineum. R. evertsievertsi had strong predilection sites for perineum, dewlap, udder/scrotum, and ears. The sex ratio of all tick species identified during this study periods was skewed towards male except for B. decolaratus. Considering the economic importance of tick and tick-borne diseases (TBDs) in the Soddo Zuria district, also in the country, there should be country wide control strategy, taking into account acaricide residues in products.
keywords: Attachment site; Cattle; Ixodidae; Prevalence, Soddo zuria woreda; Tick burden
Introduction
Ticks are obligate, blood-feeding ectoparasites of vertebrates, particularly mammals and birds. It has been estimated that about 80% of the world population of cattle is infested with ticks. The lifecycle of ticks (both Ixodids and Argasids) undergo moulting stages in their development; eggs, 6-legged larva, 8-legged nymph and adult [1]. According to the numbers of hosts, Ixodids ticks are classified as one-host ticks, two-host ticks, three-host ticks and Argasids classified as multi-host ticks. In one-host ticks, all the parasitic stages (larva, nymph, and adult) are on the same hosts; in two- host ticks, larva attach to one host, feed and moult to nymphal stage and engorged, after which they detach and moult on the ground to adult; and in three-host ticks, the larva, nymph, and adult attach to different hosts and all detach from the host after engorging, and molt on the ground. In multi-host ticks (Argasids), a large number of hosts are involved, and it is common to have five molts, each completed after engorging and detaching from the hosts [2].
Ticks are most numerous, particularly in tropical and sub-tropical regions, and their impact on animal health and production is greatest in these regions [3]. Ticks are usually relatively large and long-lived, compared to mites, surviving for up to several years [4]. Ticks belong to the phylum Arthropod, class Arachnid, and order Acari. The families of ticks parasitizing livestock are categorized into two, the Ixodidae (hard ticks) and Argasidae (soft ticks). Though sharing certain basic prosperities, they differed in many structures, behavioral, physiological, feeding, and reproduction pattern [5]. Ticks that are considered to be most important to domestic animals’ health in Africa comprise about seven genera and forty species. Among these tick genera, the main ticks found in Ethiopia are Ambylomma (40%), Boophilus (21%), Heamaphysalis (0.5%), Haylomma (1.5%), and Rhipicephalus (37%) [1]. Among these, A. varigatum and B. decoloratus are the most important and widely distributed [6].
A. coherence, A. gemma, A. lepidium, Haylomma marginatum rufipes, H. truncatum, and R. evertsi are also commonly found in Ethiopia [7,8]. Even though there were a number of studies on ticks and tick-borne diseases (TBDs) in many parts of Ethiopia, in some location, there was no previous study on tick and TBDs. Therefore, the objectives of this study were to estimate the prevalence of tick infestation of cattle in Soddo Zuria district and to identify the common tick species in Soddo Zuria district.
Materials and Methods
Description of study area
The tick survey was conducted in South Nation Nationalities and Peoples Region, Wolaita zone particularly, in Soddo Zuria woreda from November 2016 to March 2017. The town is located 380 km Southwest of Addis Ababa on the way to Arbaminch town and it has a latitude and longitude of 6°54′N 37°45′E with an elevation between 1650 and 2980 meters above sea level. The town is bounded with Damot Gale Woreda to the North, Humbo Woreda to the South, Damot Woide Woreda to East and Damot Sore Woreda to the West. The annual rainfall of the area is 1000-1200mm. The dry season extends from September to February and the rain season stays from March to August. The livestock population of the area comprised about 1,097,710 cattle; 150,383 sheep; 185,250 goats; 60,055 equines and 734,924 poultry [9].
Study population
The cattle population found in Soddo Zuria woreda was considered as a study population. The study populations were constituted in all breeds that are known as local, cross and exotic breeds depending on their respective blood level. Therefore, local breeds have pure indigenous traits, cross breeds have both indigenous and exotic traits about less than 75% and exotic breeds are considered having more than 75% of the exotic trait.
Study methodology
The selected study animal was properly restrained, and all tick samples were collected from half the body regions. Ticks were removed carefully and gently in a horizontal pull to the body surface. The collected ticks were preserved in universal bottles containing 70% ethanol and labeled with respect to predilection site, age, sex and date of collection, then transported to Wolaita Sodo Regional Veterinary Laboratory for counting and identification. The ticks were counted and subsequently identified to genus and species level by using stereomicroscope, according to standard identification keys as given by [10].
Sampling design and technique
A cross-sectional study was conducted to determine the prevalence of ticks, and identification of species of ticks collected and labeled according to predilection site. All the animals selected as sampling unit were checked for any tick infestation based upon the numbers of ticks found on the animal and the study record period. Ticks were collected from ears, heads, dewlaps, belly/flunk, udder/scrotum, perineum and legs/tails in the separated sample bottles with 70% ethanol. In addition to the attachment site of tick in different body regions, the burden of ticks based on age, sex, body condition, and breeds of animals were determined.
Sampling methods and determination of sample size
The cattle to be examined were selected by simple random sampling method, and multistage sampling strategy was used to determine appropriate sample size. The sample size was determined by using the formula given by Thrustifield [11]. The expected prevalence of Ixodidae ticks of cattle in Soddo Zuria woreda was assumed as 50%. The parameters used were 95% confidence interval and 5% desired level of precision. By substituting these values in the formula, the sample size was calculated to be 384 as shown below but, 501 animals were included in the study to increase precision.
Where,
n=Sample size;
Pexp=Expected prevalence;
d2=Expected precision which is usually 5% (0.05)
Data analysis
The data collected were entered and managed in Microsoftexcel. An intercooled SPSS 20 version software [12] statistical program was employed for the data analysis. The overall prevalence of tick was determined by dividing the number of positive animals by total sample size and was expressed as a percentage. Chi-square (X2) test was used to assess if there was a statistically significant association in tick infestation between ages, sex, breeds and body conditions.
Result
In the current survey, a total of 501 animals were included with breed distribution of local (n=308), cross (n=137), and exotic (n=56). Out of the 501 animals examined, ticks were found on 327 animals yielding an overall prevalence of 65.27%. The distribution of tick genera was identified and located in Table 1 below. The statistical analysis was done for the prevalence of tick infestation with hypothesized risk factors (age, sex, breed and body condition). There were statistically significant association with breeds (x2=11.9698, P=0.003), body conditions (x2=14.2337, P=0.001) and age (x2=9.8475, P=0.0) (Table 2). Higher tick infestation rate was seen on both poor body condition and local breeds. There were no statistical significances (P>0.05) associated with the sex of animals (Table 2). Of the total 3290 Ixodid ticks collected from seven body region of 327 cattle, four different species in three genera were identified. The tick species identified were B. decolaratus (45.47%), R. evertsi subspecies evertsi (13.98%), A. varigatum (23.04%) and A. cohaerence (17.5%) (Table 3). By considering the relative abundance of each tick species identified in the study area, B. decolaratus was the most abundant (45.47%) and R.eversi subspecies eversi were the least abundant (13.98%).
Table 1:Distribution of tick genera of cattle in the study area.
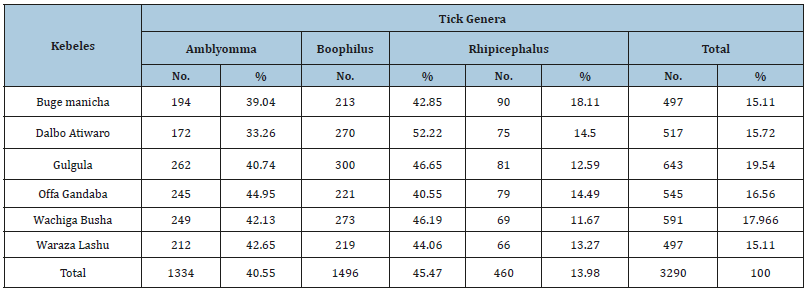
Table 2:Prevalence of tick in relation to body conditions breeds, sex and age of animals.
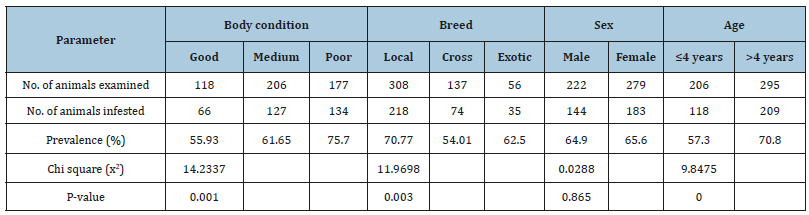
Table 3:Number of tick Species identified in half body region of cattle.
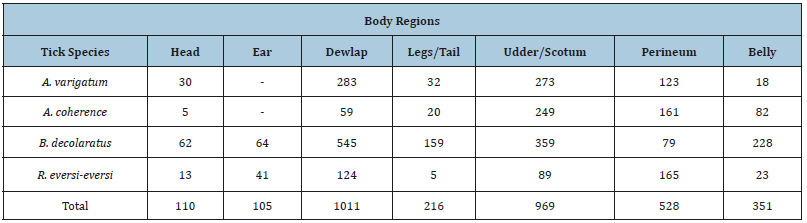
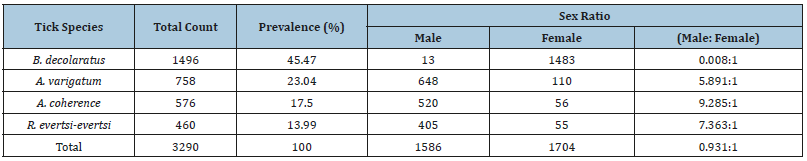
The observed proportion of tick species of Ambylomma identified during the study preferred udder/scrotum, dewlap/ brisket, perineum, belly/back, legs/tail, and head regions. The B. decolaratus preferred the attachment site such as dewlap/brisket, udder/scrotum, belly/back, legs/tail, perineum, ears, and heads regions. The Rhipicephalus species were encountered mainly in the perineum, dewlap, udder/scrotum, ears, belly/back, and head and legs/tail regions (Table 3). The numbers of ticks collected from the cattle dominated by males, but an exception was found in one host tick (B. decolaratus) in which females’ collection was higher than the males’ (Table 4).
Table 4:The distribution and sex ratio of adult tick species in the study area.
Discussion
The prevalence and distribution of the most common tick species infesting cattle are different from one area to another. In the present study, a detailed investigation was carried out to identify and determine the type of species and predilection site of ticks infesting cattle in Soddo Zuria district. Although there are different species of ticks, only four species of ticks, B. decolaratus, A. varigatum, A. coherence, and R. evertsi evertsi were identified. B. decolaratus were found to be the most abundant tick species in Soddo Zuria district with a rate of 45.47%. This finding was in agreement with the report of Sileshi et al. [13] who described that B. decolaratus was the commonest and most widespread tick in Ethiopia, collected in all administrative regions except in the Afar region.
The current finding was also in line with the report of Tamru [14] in Asela et al. [15] in Ethiopia, who reported the highest prevalence of B. decolaratus (80%) in the study areas. According to Shiferaw [16], B. decolaratus had the highest frequency in the observed area during dry seasons (January, February, and early March) in Wolaita zone. This result disagreed with the findings of Alekaw [17] at Metekel Ranch, Ethiopia showing the prevalence of 5.7%. This may be due to the geographical location and altitude factors which are 1,500 to 1,600m above sea level of Metekel Ranch. The females were abundant from September to April and transmitted Babesia bigemina to cattle, and a severe infestation can lead to tick worry, anorexia, and anemia [18]. The one-host ticks of the genus Boophilus that parasitize ruminants represent a hindrance to livestock farming in tropical and sub-tropical countries. They transmit the causative agents of Anaplasmosis (“Gall Sickness”) and Babesiosis (“Red Water”) in cattle [10].
A. varigatum was the second widespread tick species of the cattle in the current study area with a rate of 23.04%. This result disagreed with different reports done by other authors in different parts of Ethiopia such as Tessema & Gashaw [19] in Asela, Belew & Mekonnen [20] in Holeta, Seyoum [18], Mehair [21] in Awassa who as described A. varigatum as the first most abundant tick species in their study areas. The difference in result was due to the geographical location where A. varigatum was found in highest number in the highland and high rainfall, and also due to its being relatively active throughout the year in most part of Ethiopia.
A. varigatum is a widely distributed cattle tick in Ethiopia [22], and it is a potential vector of diseases caused by Cowdria ruminantium, Theleria mutan, T. velifera (“Benign Bovine Theileriosis”) and viral diseases, Nairobi sheep disease, and also aggravates the situation of bovine dermatophilosis (Dermtophilus congolence) [13]. The study conducted in Wolaita zone by Dessie & Getachew [23] agreed with this study that shows A. varigatum was the second abundant tick species at Highland and Midland, and the first abundant in the lowland during the wet period. This variation may be due to the change in environmental conditions, with the result of global warming that highly affects the ecology of ticks. Change in temperature and rainfall have been reported to affect the distribution of diseases of vectors and tick-borne diseases [2].
A. cohaerence was the third abundant tick species with a rate of 17.5% in the study area. Amblyomma coherence is the most prevalent and abundant tick on cattle [24]. According to [25], A. cohaerence was also the third abundant tick species in the Bench Maji zone with a prevalence of 4.2% in the area. The prevalence of A. cohaerence is alarmingly important as this tick has been reported as a vector for C. ruminantium which is the causative agent of cowdriosis (“Heartwater”) [13]. Moreover, Amblyomma coherence transmits Ehrlichiosis, but less important vector than A. varigatum. Another report indicated a spontaneous infection of Amblyomma coherence by Rickettsia conorii in Ethiopia [25,26].
R. evertsi-evertsi was found to be the least abundant with a rate of 13.98% tick species in this study area. The native distribution of R. evertsi-evertsi in Ethiopia seems to be connected with middle height dry Savannas and steppes, in association with zebra and ruminant and it is widely distributed throughout Ethiopia [20]. This tick species shows no apparent preference for particular altitude, rainfall zone or seasons [24]. According to Sileshi et al. [13], R. evertsi-evertsi was collected throughout their study period, with the peak of abundance in January coinciding with the beginning of the rainy season and they also described that the discovery of this tick in that area was in line with its widespread occurrence in most parts of the country. The occurrence of this species in and around the Wolaita zone was also reported by Dessie & Getachew [23]. R. evertsi-evertsi has short mouthparts with which to feed on a soft area. As a result, it is a possible vector of Babesia, Rickettsia, and Theleria [27].
Ticks are known to be distributed in different parts of the host’s body. In this study, the main infestation site of ticks in the body of hosts was dewlap, udder/scrotum, perineum, and belly. A variety of factors such as host density, the interaction between tick species, time and season, and inaccessibility for grooming determined the attachment site of the ticks on the skins [28]. The predilection sites found in this study were in line with those reported by Seyoum [29] and Behailu [30] in their study conducted in North Wollo zone and Asela, respectively.
In the current study, different animal-related risk factors were studied to determine whether there was a significant variation in tick infestation between and among different groups of animals with suspected risk factors. The proportion of tick infestation was higher in adult animals as compared to young animals. As a result, there was a statistically significant association (p<0.05), and the higher proportion may be due to outdoor management and longdistance movement of adult animals to search for food and water compared to younger animals, so the chance of exposure was higher. This finding was also in agreement with the finding of Feseha [31], Tessema & Gashaw [19] and Belew & Mekonnen [20] who stated a higher proportion in adult cattle.
There was a statistically non-significant association (p>0.05) in the infestation rate among different sex groups, where the higher infestation was recorded in female animals compared to their counterparts. Higher infestation in the female might be due to seasonal variation in some hormones like prolactin and progesterone at a higher level and stressors of production (such as pregnancy and lactation) that make female animals more susceptible than male [32,33]. It may also found that immunecompromised animals acquire high tick infestation in more over the stress of production such as pregnancy and lactation make the females more susceptible to such infestation. This result disagreed with the previous work done by Hussen [34] in Bako.
The proportion of tick infestation was higher in poor body conditioned (75.7%) as compared to medium body conditioned (61.65%) and good body conditioned animals (55.93%). This was due to the fact that poor body conditioned animals having reduced immunity and are exposed to any kind of disease when grazing on the field. The result of the current study agrees with Okelly & Seifert [35] who reported that the dietary deficiencies that influence the breakdown of tick resistance. The observation indicates that poor body conditioned animals are less resistant to tick infestation and lack enough body potential to build resistance with age advancement. Several authors have reported that high infestation of tick results in poor body condition due to the consumption of the high amount of blood and fluids by those ticks [36].
According to Aerts & Neshem [37] who reported the British cattle breeds having the lowest body condition score under tropical conditions had the highest infestation of ticks. Seid [38], Southerest [36] and Bianchi et al. [39] reported that tick load on the animal might be affected by breed and nutritional stress. Ultimately, these factors affect general body condition, which in turn affects blood composition, respiratory rate, appetite and eventually leads to poorer body condition score. However, the well-fed animals were very resistant to any kind of diseases when they grazed in the field or are kept at home. The fact that more tick burden was recorded in both local and exotic breeds compared to cross cattle.
The current finding agrees with the report by Belew & Mekonnen [20] that revealed the presence of tick infestation in local breeds were very high with the prevalence of 44.96% (n=223), while in cross breeds and Jersey, the prevalence were 15.83% (n=57), and 8.50% (n=30) respectively. The significant variation in tick infestation of cattle of different breeds may be attributed to the different management system, lack of supplementary feeding for local breeds, or lack of control measures against tick on local breeds. Furthermore, it can be assumed that it might be due to the lack of interest of farmers for local breeds as well as taking more care to cross and exotic breeds than local breeds.
The male to female ratios of B. decolaratus, A. varigatum, R. evertsi-evertsi, and A. cohaer were similar to previous reports of Seyoum [29] and Solomon et al. [28]. Except for B. decolaratus, all other species tick’s males outnumbered females because males normally remain on the host longer than females. Fully engorged female tick drops off to the ground to lay eggs while males tend to remain on the host up to several months to continue feeding and mating with other females on the host before dropping off [28]. The females of B. decolaratus outnumbered males in this study might be probably due to the small size of the male which may not be seen during collection [19].
Conclusion and Recommendation
Variable information on tick species distribution and dynamics are very essential to assess the economic loss encountered due to tick infestation and also to identify the appropriate measure of tick control. Among ectoparasites, ticks cause the greatest economic loss in livestock population either by transmitting a wide variety of TBDs or by affecting the health of animals as well as the quality of hiding and skins. The important and abundant tick species investigated in the study area were B. decolaratus, A. varigatum A. cohaerence, and R. evertsi-evertsi. The study indicated that there was a high burden of ticks in the area. However, the attention given to controlling the infestation had not been sufficient.
The control methods necessary for tick and TBDs were a selection of tick resistance cattle, acaricides treatment, appropriate livestock management, evaluation and incorporation of traditional practices or remedies that appear to be of value. In general, the distribution of ticks are not fixed but are determined by a complex interaction of factors such as climate, host density, host susceptibility, grazing habits, and pasture-herd management. Therefore, an effective tick control program should be formulated and implemented based on the distribution pattern of ticks and factors responsible for their distribution.
In light of the above conclusion the following recommendations are forwarded:
A. Tick control program (Application of Acaricide) should be continued with an increasing frequency of application in wet months.
B. Detection of acaricide resistance tick species which are economically important since limited types of acaricide was used in the area.
C. More attention should be given to the selection of resistant cattle breeds and types, and good performance with regards to the production of local breeds.
Appropriate pasture management in communal grazing area is recommended.
References
- Minjauw B, McLeod A (2003) Tick-borne diseases and poverty. The impact of ticks and tick-borne diseases on livestock owners in India and Eastern health program center for tropical veterinary medicine. University of Edinburgh, UK, pp. 24-57.
- Taylor MA, Coop RH, Wall RL (2007) Veterinary parasitology. (3rd edn), Black Well Publishing, London, pp. 679-712.
- Lefebvre PC, Blancou J, Chermette R, Uilenberg G (2010) Infectious and parasitic diseases of livestock 1: 93-128.
- Kettle DS (1995) Medical and veterinary entomology. (2nd edn), CAB international, UK, pp. 420-460.
- Urquhart GM, Armour J, Dunean JI, Dunn AM, Jennings FW (1996) Veterinary parasitology. (2nd edn), Black Well Science. The University of Glaskow, Scotland, UK, pp. 181-188.
- Abebaw G (2004) Seasonal dynamics and host preference of Boophilus decolaratus on naturally infested cattle in Jimma zone, South Western Ethiopia. Ethiopia Vet J 18(1): 19-28.
- Solomon G, Kaaya GP (1996) Studies on the developmental and survival of Rhipicephalus pulchelu and Amblyoma gemma under field condition, Ethiopia.
- Pegram PG, Tatchell RJ, De Castro JJ, Chizyuka MJ, Mc Cosker PG, et al. (2004) Tick control: New concepts.
- Dado WS (2013) Wolaita soddo district agricultural development office.
- Walker AA, Bouatour A, Camicas JL, Estadapena AA, Harok IG, et al. (2003) Ticks of domestic animals in Africa: A guide to identification species. The University of Edinburgh, UK, pp. 67-80.
- Thrustfield M (1995) Veterinary Epidemiology. (2nd edn), UK Black Well Science Ltd., pp. 182-198.
- STATA Corporation (2001) Stata statistical software Release 7.0. College Station, Texas.
- Sileshi M, Pegram RG, Solomon G, Abebe M, Yilma J, et al. (2007) A synthesis of review of Ixodids (Acari: Ixodidae) and Argas (Acari: Argasidae) ticks in Ethiopia and their possible role in diseases transmission. Ethiopia Vet J 2: 1-22.
- Tamru T (2008) Survey of Bovine tick species in and around Asela Town, DVM Thesis, School of veterinary medicine, Jimma University, Jimma, Ethiopia.
- Teshome Y, Feseha G, Wakjira A, Tsega T (1995) Preliminary observation on ticks: Seasonal dynamics and resistance of three indigenous and three cross-breed cattle in Ethiopia. Bull Anim Health Prod Afr 43: 105-114.
- Shiferaw D (2005) Cattle tick dynamics in different agro-ecological zones of Wolaita, Southern Ethiopia. Master degree thesis. Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia, pp. 1-137.
- Alekaw S (1998) Distribution of tick and tick-borne diseases at Metekel Ranch. Ethiopia Vet J 4(1): 30.
- Seyoum Z (2005) Distribution and the host-parasite relationship of Ixodids ticks in Eastern Amhara, Kombolcha Regional Veterinary Laboratory, Kombolcha, Ethiopia, pp. 34-47.
- Tessema T, Gashaw A (2010) Prevalence of ticks on local and crossbreed cattle in and around Asela Town, South East, Ethiopia, Amber Animal Health Department, East Gojam, Ethiopia Vet J 14(2): 79-89.
- Belew T, Mekonnen A (2011) Distribution of Ixodidae ticks on cattle in and around Holeta Town, Ethiopia. Global Veterinaria 7(6): 527-531.
- Mehari B (2004) Distribution of livestock tick species in Awassa Area. DVM Thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia.
- Morel P (1980) Study on Ethiopian ticks (Acaridae: Ixodidae). (1st edn), The republic of France, ministry of foreign affairs, French vet mission, Addis Ababa, Ethiopia, pp. 15-183.
- Dessie S, Getachew A (2006) Cattle tick dynamics in different agroecological zones of Wolaita, Southern Ethiopia. Ethiopia Vet J 10(2): 85- 99.
- Pegram RG, Hoogstral, Wassef HY (1981) Ticks (Acari: Ixodidae) of Ethiopia. Distribution, ecology, and the relation of species infesting livestock. Bull Entomol Res 71: 335-359.
- Tesfaheywet ZS, Simeon HO (2012) Prevalence of ectsoparasite infestations of cattle in Bench Maji zone, Southwest Ethiopia. College of Vet Med, Haramaya University, Ethiopia.
- Morel P (1989) Manual tropical veterinary parasitological. CBA International, UK, pp. 299-460.
- Kettle DS (1995) Medical and veterinary entomology. (2nd edn) CAB international, UK, pp. 420-460.
- Solomon G, Night M, Kaaya GP (2001) Seasonal variation of ticks on calves at Sabetha, Western Shewa zone. Ethiopia. Vet J 7(1 & 2): 17-30.
- Seyoum Z (2001) Study of tick and TBDs on cattle at Girran valley in the North Wollo Zone. Proceeding the Ethiopia Vet Assoc, p. 15.
- Behailu A (2004) A study of ticks and tick-borne protozoans in cattle at Assela, Arsi Zone (DVM thesis). FVM, AAU, Debre Zeit, Ethiopia.
- Feseha B (1997) Species composition and distribution ofIxodid ticks in Eastern Harerghiea, Ethiopia. Agr Sci 16: 37-51.
- Etter E, Chartier C, Lefrileux Y, Borgid LP (1999) The influence of nutrition on the periparturient rise in fecal egg counts in dairy goats. Revue de Med Vet 150: 975-980.
- Lloyd S (1983) Effect of pregnancy and lactation up on infection. Vet Immunol Immunop, pp. 4153-4176.
- Hussen Y (2009) A preliminary survey of cattle tick species and burden in and around Bako Town. DVM Thesis, School of Veterinary Medicine Jimma University, Jimma, Ethiopia. International Center for Insect Physiology and Zone Ecology press, Nairobi, Kenya, pp. 1-124.
- Okelly JC, Seifert GW (1969) Relationship between resistances to Boophilus microplus, nutritional status, blood composition in shorthorn X Hereford cattle. Aust J Bio SA 22: 1497-1509.
- Southeast RW (1983) Management of Arthropods and Parasitism in Livestock. In: Dunsmare JP (Ed.), Tropical Parasitosis and parasitic zoonoses, Murdon University, Perth, Australia, pp. 41-56.
- Aerts JM, Neshem ON (1999) Florida beef cattle external pest control survey. UF Pesticide Information Office: Report in Production.
- Seid B (2004) Survey of cattle tick species in and around Mezan Teferi, Bench Maji zone of SNNPR. DVM Thesis, Faculty of veterinary medicine, Addis Ababa University, Debre Zeit, Ethiopia.
- Bianchi MW, Barre VS, Messad (2003) Factors related to cattle infestation level and resistance to acaricides in Boophyilus microplus tick populations in New Caledonia. Vet Parasitol 112(1-2): 75-89.




No comments:
Post a Comment