Investigation of the Degradation Stages of Archaeological and Historical Silk Textiles: An ATR-FTIR Spectroscopic Study by Sevim A* in Archaeology & Anthropology:Open Access_ American Journal of Archaeology
Abstract
Silk
fibers are found in many important historic textiles and artefacts. Raw silk is
a proteinaceous fiber consists of two major proteins: The main fibrous
component fibroin, and amorphous protein sericin. In commercial use, sericin is
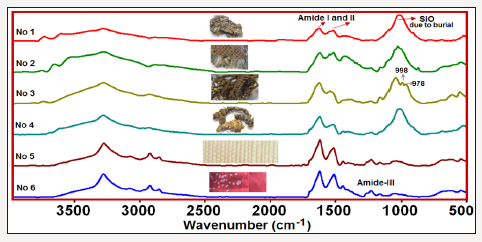
normally removed by degumming. Fourier transform infrared (FTIR) spectroscopy
is a powerful tool for fiber identification and can provide both qualitative
and quantitative information about protein conformations. The amino acid
composition of fibroin and sericin significantly differs from each other, thus
they can be easily differentiated by IR spectroscopy. In this study some
archaeological silk textile specimens, obtained during the excavations in
Ancien Ainos (Enez), one of the most important archaeological sites in Turkey
together with 150 years old and new Bombyx mori silk textiles were investigated
using Attenuated Total Reflectance-Fourier transform infrared (FTIR) spectroscopy. The main aim of
our research was to investigate conformational changes of silk protein, caused
by ageing depending on the environmental conditions. It was found that
structural transformations from β-sheet domain to β-turn and disordered
conformations occur due to degradation. By aging, the changes in molecular
structure and the formation of oxidative moieties were investigated. The
crystallinity and oxidation degrees of the silk fibroin were defined by the
investigations of the ratios of integrated intensities of β-Sheet to disordered
structure and the integrated intensity ratios of Amide I/amide II, respectively.
https://crimsonpublishers.com/aaoa/fulltext/AAOA.000573.php
Crimson
Publishers: https://crimsonpublishers.com/
For
more articles in American Journal of
Archaeology,
Please
click on below link: https://crimsonpublishers.com/aaoa/



No comments:
Post a Comment